MondoA and AKI and AKI-to-CKD Transition
- PMID: 38819935
- PMCID: PMC11387036
- DOI: 10.1681/ASN.0000000000000414
MondoA and AKI and AKI-to-CKD Transition
Abstract
Key Points:
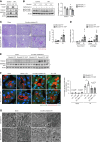
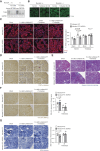
The expression of MondoA was decreased in the renal tubules of patients with CKD.
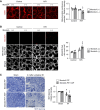
Genetic ablation of MondoA in proximal tubules inhibited autophagy and increased vulnerability to AKI through increased expression of Rubicon.
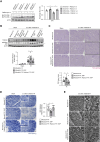
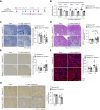
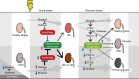
MondoA ablation during the recovery phase after ischemia-reperfusion aggravated kidney injury through downregulation of the transcription factor EB-peroxisome proliferator-activated receptor-γ coactivator-1α axis.
Background: Elderly individuals and patients with CKD are at a higher risk of AKI. The transcription factor MondoA is downregulated in the kidneys of aged individuals or patients with AKI; however, its roles in AKI development and the AKI-to-CKD transition remain unknown.
Methods: We investigated the expression of MondoA in human kidney biopsy samples, ischemia-reperfusion–injured (IRI) mouse kidneys, and cultured proximal tubular epithelial cells under hypoxia/reoxygenation. The role of MondoA during the initial and recovery phases after IRI was evaluated using proximal tubule–specific MondoA knockout mice and MondoA-deficient proximal tubular epithelial cells. Furthermore, we explored the involvement of Rubicon and transcription factor EB (TFEB), both of which are downstream factors of MondoA.
Results: MONDOA expression was decreased in the renal tubules of patients with CKD. In mouse kidneys, MondoA expression was decreased under ischemia, whereas its expression was increased during reperfusion. Genetic ablation of MondoA in proximal tubular epithelial cells inhibited autophagy and increased vulnerability to AKI through increased expression of Rubicon. Ablation of Rubicon in MondoA-deficient IRI kidneys activated autophagy and protected mitochondrial function. MondoA ablation during the recovery phase after ischemia-reperfusion aggravated kidney injury through downregulation of the TFEB-peroxisome proliferator-activated receptor-γ coactivator-1α axis. Pharmacological upregulation of TFEB contributed to maintaining mitochondrial biogenesis and increased peroxisome proliferator-activated receptor-γ coactivator-1α transcription.
Conclusions: Our findings demonstrate that MondoA protected against vulnerability to AKI by maintaining autophagy and subsequently supporting mitochondrial function to prevent progression to CKD.
Keywords: AKI; CKD; fibrosis; hypoxia; ischemia-reperfusion; kidney biopsy; kidney tubule; mitochondria; molecular biology; transcription factors.
Conflict of interest statement
Disclosure forms, as provided by each author, are available with the online version of the article at
Figures



References
Grants and funding
- JP22gm1410014/AMED
- 21K08276/a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology in Japan
- 22K16240/a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology in Japan
- 21H02935/a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology in Japan
- None/Novo Nordisk Pharma
LinkOut - more resources
Full Text Sources

