Triple Radical Sorting: Aryl-Alkylation of Alkenes
- PMID: 38820134
- PMCID: PMC11610504
- DOI: 10.1021/jacs.4c05744
Triple Radical Sorting: Aryl-Alkylation of Alkenes
Abstract
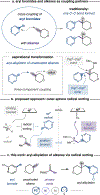
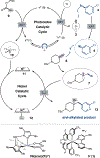
The cross-coupling of aryl bromides with alkenes can provide access to diverse combinatorial chemical space. Two-component couplings between these partners are well-known, but three-component aryl-functionalizations of unactivated alkenes remain underdeveloped. In particular, the aryl-alkylation of unactivated alkenes would allow for rapid construction of molecular complexity and the expedient exploration of a pharmaceutically relevant and C(sp3)-rich structural landscape. Herein, we report a general approach toward the aryl-alkylation of alkenes through a triple radical sorting mechanism. Over the course of the reaction, a high energy aryl radical, a primary radical, and a hindered alkyl radical are simultaneously formed. Through mediation by a nickel-based catalyst, the three radicals are sorted into productive bond-forming pathways toward the efficient aryl-alkylation of alkenes. A wide range of electronically and sterically differentiated alkenes and aryl radical precursors can be used to access complex scaffolds. This method was further applied to the synthesis of highly substituted semisaturated fused heterocycles.
Conflict of interest statement
The authors declare the following competing financial interest(s): D.W.C.M. declares a competing financial interest with respect to the integrated photoreactor.
Figures
References
-
- Lovering F; Bikker J; Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem 2009, 52 (21), 6752–6756. - PubMed
-
- Lovering F Escape from Flatland 2: Complexity and Promiscuity. Med. Chem. Commun 2013, 4 (3), 515.
-
- Everson DA; Shrestha R; Weix DJ Nickel-Catalyzed Reductive Cross-Coupling of Aryl Halides with Alkyl Halides. J. Am. Chem. Soc 2010, 132 (3), 920–921. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources