HSDL2 links nutritional cues to bile acid and cholesterol homeostasis
- PMID: 38820148
- PMCID: PMC11141617
- DOI: 10.1126/sciadv.adk9681
HSDL2 links nutritional cues to bile acid and cholesterol homeostasis
Abstract
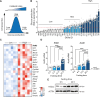
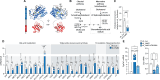
In response to energy and nutrient shortage, the liver triggers several catabolic processes to promote survival. Despite recent progress, the precise molecular mechanisms regulating the hepatic adaptation to fasting remain incompletely characterized. Here, we report the identification of hydroxysteroid dehydrogenase-like 2 (HSDL2) as a mitochondrial protein highly induced by fasting. We show that the activation of PGC1α-PPARα and the inhibition of the PI3K-mTORC1 axis stimulate HSDL2 expression in hepatocytes. We found that HSDL2 depletion decreases cholesterol conversion to bile acids (BAs) and impairs FXR activity. HSDL2 knockdown also reduces mitochondrial respiration, fatty acid oxidation, and TCA cycle activity. Bioinformatics analyses revealed that hepatic Hsdl2 expression positively associates with the postprandial excursion of various BA species in mice. We show that liver-specific HSDL2 depletion affects BA metabolism and decreases circulating cholesterol levels upon refeeding. Overall, our report identifies HSDL2 as a fasting-induced mitochondrial protein that links nutritional signals to BAs and cholesterol homeostasis.
Figures

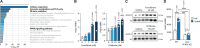



References
-
- Saltiel A. R., You are what you secrete. Nat. Med. 7, 887–888 (2001). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

