Synthesis of Complex Tetracyclic Fused Scaffolds Enabled by (3 + 2) Cycloaddition
- PMID: 38820198
- PMCID: PMC11187634
- DOI: 10.1021/acs.orglett.4c01269
Synthesis of Complex Tetracyclic Fused Scaffolds Enabled by (3 + 2) Cycloaddition
Abstract
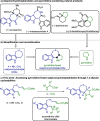
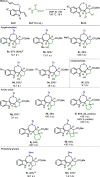
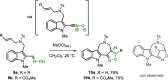
We describe the single-step formation of complex tetracyclic fused scaffolds enabled by (3 + 2) cycloaddition of azomethine ylides. Various indoles, N-protecting groups, and amino acids are well tolerated. The products are obtained in a catalyst-free manner with moderate to excellent yield and high diastereoselectivity. Representing a new scaffold that is not yet found in nature, the construction of pyrrolidine-fused cyclohepta-, azepino-, or oxepinoindoles could be found valuable in the synthesis of new pseudo-natural products.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Reymond J.-L.; van Deursen R.; Blum L. C.; Ruddigkeit L. Chemical Space as a Source for New Drugs. Med. Chem. Commun. 2010, 1 (1), 30–38. 10.1039/c0md00020e. - DOI
LinkOut - more resources
Full Text Sources

