Novel Liver Injury Phenotypes and Outcomes in Clinical Trial Participants with Pulmonary Hypertension
- PMID: 38820270
- PMCID: PMC11531102
- DOI: 10.1164/rccm.202311-2196OC
Novel Liver Injury Phenotypes and Outcomes in Clinical Trial Participants with Pulmonary Hypertension
Abstract
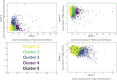
Rationale: Pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH) cause right ventricular dysfunction, which can impact other solid organs. However, the profiles and consequences of hepatic injury resulting from PAH and CTEPH have not been well studied. Objectives: We aimed to identify underlying patterns of liver injury in a cohort of patients with PAH and CTEPH enrolled in 15 randomized clinical trials conducted between 1998 and 2014. Methods: We used unsupervised machine learning to identify liver injury clusters in 13 trials and validated the findings in two additional trials. We then determined whether these liver injury clusters were associated with clinical outcomes or treatment effect heterogeneity. Measurements and Main Results: Our training dataset included 4,219 patients and our validation dataset included 1,756 patients with serum total bilirubin, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, and albumin data. Using k-means clustering, we identified phenotypes with no liver injury, hepatocellular injury, cholestatic injury, and combined injury patterns. Patients in the cholestatic injury liver cluster had the shortest time to clinical worsening and the highest risk of mortality. The cholestatic injury group also experienced the greatest placebo-corrected treatment effect on 6-minute-walk distance. Randomization to the experimental arm transitioned patients to a healthier liver status. Conclusions: Liver injury was associated with adverse outcomes in patients with PAH and CTEPH. Randomization to active treatment had beneficial effects on liver health compared with placebo. The role of liver disease (often subclinical) in determining outcomes warrants prospective studies.
Keywords: liver injury phenotypes; machine learning; pulmonary hypertension.
Figures




Update of
-
Novel Liver Injury Phenotypes and Outcomes in Pulmonary Arterial Hypertension.medRxiv [Preprint]. 2023 Sep 30:2023.09.28.23296316. doi: 10.1101/2023.09.28.23296316. medRxiv. 2023. Update in: Am J Respir Crit Care Med. 2024 Oct 15;210(8):1045-1056. doi: 10.1164/rccm.202311-2196OC. PMID: 37808731 Free PMC article. Updated. Preprint.
Comment in
-
Beyond the Lung: Viewing Treatment Response through the Liver in Pulmonary Arterial Hypertension.Am J Respir Crit Care Med. 2024 Oct 15;210(8):976-978. doi: 10.1164/rccm.202405-1017ED. Am J Respir Crit Care Med. 2024. PMID: 38913580 Free PMC article. No abstract available.
References
-
- Rosenkranz S, Howard LS, Gomberg-Maitland M, Hoeper MM. Systemic consequences of pulmonary hypertension and right-sided heart failure. Circulation . 2020;141:678–693. - PubMed
-
- Marcus JT, Westerhof BE, Groeneveldt JA, Bogaard HJ, de Man FS, Vonk Noordegraaf A. Vena cava backflow and right ventricular stiffness in pulmonary arterial hypertension. Eur Respir J . 2019;54:1900625. - PubMed
-
- Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. ESC/ERS Scientific Document Group 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J . 2022;43:3618–3731. - PubMed
-
- Scott JV, McClelland R, Weinberg E, Al-Namaani N, Baird G, Holmes J, et al. Unsupervised machine learning for identification of cardiohepatic syndrome in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension [abstract] Am J Respir Crit Care Med . 2022;205:A5304.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

