Randomized study of induction with bendamustine-rituximab ± bortezomib and maintenance with rituximab ± lenalidomide for MCL
- PMID: 38820500
- PMCID: PMC11561533
- DOI: 10.1182/blood.2024023962
Randomized study of induction with bendamustine-rituximab ± bortezomib and maintenance with rituximab ± lenalidomide for MCL
Abstract
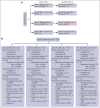
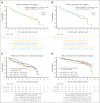
Although initial therapy of mantle cell lymphoma (MCL) is not standardized, bendamustine plus rituximab (BR) is commonly used in older patients. Rituximab (R) maintenance after induction is often used. Thus, the open-label, randomized phase 2 ECOG-ACRIN Cancer Research Group E1411 trial was designed to test 2 questions: (1) does addition of bortezomib to BR induction (BVR) and/or (2) addition of lenalidomide to rituximab (LR) maintenance improve progression-free survival (PFS) in patients with treatment-naïve MCL? From 2012 to 2016, 373 previously untreated patients, 87% aged ≥60 years, were enrolled in this trial. At a median follow-up of 7.5 years, there is no difference in the median PFS of BR compared with BVR (5.5 vs 6.4 years; hazard ratio [HR], 0.90; 90% confidence interval [CI], 0.70-1.16). There were no unexpected additional toxicities with BVR treatment compared with BR, with no impact on total dose/duration of treatment received. Independent of the induction treatment, addition of lenalidomide did not significantly improve PFS, with median PFS in R vs LR (5.9 vs 7.2 years; HR, 0.84; 90% CI, 0.62-1.15). Most patients completed the planned 24 cycles of LR at the scheduled dose. In summary, adding bortezomib to BR induction does not prolong PFS in treatment-naïve MCL, and LR maintenance was not associated with longer PFS compared with R alone after BR. Nonetheless, the >5-year median PFS outcomes in this prospective cooperative group trial indicate the efficacy of BR followed by R maintenance as highly effective initial therapy for older patients with MCL. This trial was registered at www.clinicaltrials.gov as #NCT01415752.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflict-of-interest disclosure: P.M. consulted for AstraZeneca, Bayer, BeiGene, Bristol Myers Squibb (BMS), Epizyme, Gilead, Janssen, Karyopharm, Merck, MorphoSys, Regeneron, and Takeda. B.G.T. holds a patent with/receives royalties from Mustang Bio; received research funding from Mustang Bio, BMS/Celgene; and serves as a consultant for Mustang Bio and Proteios Technologies. E.D.H. received research funding from Eli Lilly; serves as consulted for Novartis; and holds stock options in Abcon therapeutics. S.D. holds equity in Data Driven Bioscience. The remaining authors declare no competing financial interests.
Figures


Comment in
-
More is not always better.Blood. 2024 Sep 5;144(10):1033-1035. doi: 10.1182/blood.2024025289. Blood. 2024. PMID: 39235798 No abstract available.
References
-
- Armitage JO, Longo DL. Mantle-cell lymphoma. N Engl J Med. 2022;386(26):2495–2506. - PubMed
-
- NCCN clinical practice guidelines in oncology (NCCN guidelines®) for B-cell lymphoma version 6.2023. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf
-
- Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203–1210. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UG1 CA233331/CA/NCI NIH HHS/United States
- UG1 CA189971/CA/NCI NIH HHS/United States
- UG1 CA233320/CA/NCI NIH HHS/United States
- UG1 CA233234/CA/NCI NIH HHS/United States
- U24 CA196172/CA/NCI NIH HHS/United States
- UG1 CA232760/CA/NCI NIH HHS/United States
- UG1 CA189828/CA/NCI NIH HHS/United States
- UG1 CA189863/CA/NCI NIH HHS/United States
- U10 CA180863/CA/NCI NIH HHS/United States
- P01 CA214274/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- UG1 CA233328/CA/NCI NIH HHS/United States
- UG1 CA233339/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- UG1 CA233277/CA/NCI NIH HHS/United States
- UG1 CA233253/CA/NCI NIH HHS/United States
- UG1 CA189957/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

