A panel of phenotypically and genotypically diverse bioluminescent:fluorescent Trypanosoma cruzi strains as a resource for Chagas disease research
- PMID: 38820564
- PMCID: PMC11168640
- DOI: 10.1371/journal.pntd.0012106
A panel of phenotypically and genotypically diverse bioluminescent:fluorescent Trypanosoma cruzi strains as a resource for Chagas disease research
Abstract
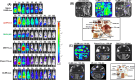
Chagas disease is caused by Trypanosoma cruzi, a protozoan parasite that displays considerable genetic diversity. Infections result in a range of pathological outcomes, and different strains can exhibit a wide spectrum of anti-parasitic drug tolerance. The genetic determinants of infectivity, virulence and therapeutic susceptibility remain largely unknown. As experimental tools to address these issues, we have generated a panel of bioluminescent:fluorescent parasite strains that cover the diversity of the T. cruzi species. These reporters allow spatio-temporal infection dynamics in murine models to be monitored in a non-invasive manner by in vivo imaging, provide a capability to detect rare infection foci at single-cell resolution, and represent a valuable resource for investigating virulence and host:parasite interactions at a mechanistic level. Importantly, these parasite reporter strains can also contribute to the Chagas disease drug screening cascade by ensuring that candidate compounds have pan-species in vivo activity prior to being advanced into clinical testing. The parasite strains described in this paper are available on request.
Copyright: © 2024 Olmo et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

