Pseudomonas aeruginosa senses and responds to epithelial potassium flux via Kdp operon to promote biofilm
- PMID: 38820569
- PMCID: PMC11168685
- DOI: 10.1371/journal.ppat.1011453
Pseudomonas aeruginosa senses and responds to epithelial potassium flux via Kdp operon to promote biofilm
Abstract
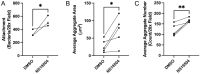
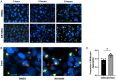
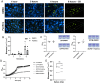
Mucosa-associated biofilms are associated with many human disease states, but the host mechanisms promoting biofilm remain unclear. In chronic respiratory diseases like cystic fibrosis (CF), Pseudomonas aeruginosa establishes chronic infection through biofilm formation. P. aeruginosa can be attracted to interspecies biofilms through potassium currents emanating from the biofilms. We hypothesized that P. aeruginosa could, similarly, sense and respond to the potassium efflux from human airway epithelial cells (AECs) to promote biofilm. Using respiratory epithelial co-culture biofilm imaging assays of P. aeruginosa grown in association with CF bronchial epithelial cells (CFBE41o-), we found that P. aeruginosa biofilm was increased by potassium efflux from AECs, as examined by potentiating large conductance potassium channel, BKCa (NS19504) potassium efflux. This phenotype is driven by increased bacterial attachment and increased coalescence of bacteria into aggregates. Conversely, biofilm formation was reduced when AECs were treated with a BKCa blocker (paxilline). Using an agar-based macroscopic chemotaxis assay, we determined that P. aeruginosa chemotaxes toward potassium and screened transposon mutants to discover that disruption of the high-sensitivity potassium transporter, KdpFABC, and the two-component potassium sensing system, KdpDE, reduces P. aeruginosa potassium chemotaxis. In respiratory epithelial co-culture biofilm imaging assays, a KdpFABCDE deficient P. aeruginosa strain demonstrated reduced biofilm growth in association with AECs while maintaining biofilm formation on abiotic surfaces. Furthermore, we determined that the Kdp operon is expressed in vivo in people with CF and the genes are conserved in CF isolates. Collectively, these data suggest that P. aeruginosa biofilm formation can be increased by attracting bacteria to the mucosal surface and enhancing coalescence into microcolonies through aberrant AEC potassium efflux sensed by the KdpFABCDE system. These findings suggest host electrochemical signaling can enhance biofilm, a novel host-pathogen interaction, and potassium flux could be a therapeutic target to prevent chronic infections in diseases with mucosa-associated biofilms, like CF.
Copyright: © 2024 Rapsinski et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
The DeltaF508-CFTR mutation results in increased biofilm formation by Pseudomonas aeruginosa by increasing iron availability.Am J Physiol Lung Cell Mol Physiol. 2008 Jul;295(1):L25-37. doi: 10.1152/ajplung.00391.2007. Epub 2008 Mar 21. Am J Physiol Lung Cell Mol Physiol. 2008. PMID: 18359885 Free PMC article.
-
Dispersal of Epithelium-Associated Pseudomonas aeruginosa Biofilms.mSphere. 2020 Jul 15;5(4):e00630-20. doi: 10.1128/mSphere.00630-20. mSphere. 2020. PMID: 32669459 Free PMC article.
-
Staphylococcus aureus Biofilm Growth on Cystic Fibrosis Airway Epithelial Cells Is Enhanced during Respiratory Syncytial Virus Coinfection.mSphere. 2018 Aug 15;3(4):e00341-18. doi: 10.1128/mSphere.00341-18. mSphere. 2018. PMID: 30111629 Free PMC article.
-
Microenvironmental characteristics and physiology of biofilms in chronic infections of CF patients are strongly affected by the host immune response.APMIS. 2017 Apr;125(4):276-288. doi: 10.1111/apm.12668. APMIS. 2017. PMID: 28407427 Review.
-
Airway biofilms: implications for pathogenesis and therapy of respiratory tract infections.Treat Respir Med. 2005;4(4):241-53. doi: 10.2165/00151829-200504040-00003. Treat Respir Med. 2005. PMID: 16086598 Review.
Cited by
-
EF-hand calcium sensor, EfhP, controls transcriptional regulation of iron uptake by calcium in Pseudomonas aeruginosa.mBio. 2024 Nov 13;15(11):e0244724. doi: 10.1128/mbio.02447-24. Epub 2024 Oct 22. mBio. 2024. PMID: 39436074 Free PMC article.
-
Effects of highly effective modulator therapy on the dynamics of the respiratory mucosal environment and inflammatory response in cystic fibrosis.Pediatr Pulmonol. 2024 May;59(5):1266-1273. doi: 10.1002/ppul.26898. Epub 2024 Feb 14. Pediatr Pulmonol. 2024. PMID: 38353361 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

