Disruption of common ocular developmental pathways in patient-derived optic vesicle models of microphthalmia
- PMID: 38821055
- PMCID: PMC11390689
- DOI: 10.1016/j.stemcr.2024.05.001
Disruption of common ocular developmental pathways in patient-derived optic vesicle models of microphthalmia
Abstract
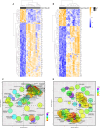
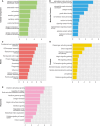
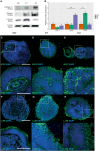
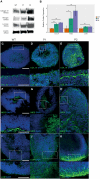
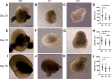
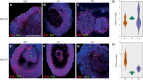
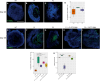
Genetic perturbations influencing early eye development can result in microphthalmia, anophthalmia, and coloboma (MAC). Over 100 genes are associated with MAC, but little is known about common disease mechanisms. In this study, we generated induced pluripotent stem cell (iPSC)-derived optic vesicles (OVs) from two unrelated microphthalmia patients and healthy controls. At day 20, 35, and 50, microphthalmia patient OV diameters were significantly smaller, recapitulating the "small eye" phenotype. RNA sequencing (RNA-seq) analysis revealed upregulation of apoptosis-initiating and extracellular matrix (ECM) genes at day 20 and 35. Western blot and immunohistochemistry revealed increased expression of lumican, nidogen, and collagen type IV, suggesting ECM overproduction. Increased apoptosis was observed in microphthalmia OVs with reduced phospho-histone 3 (pH3+) cells confirming decreased cell proliferation at day 35. Pharmacological inhibition of caspase-8 activity with Z-IETD-FMK decreased apoptosis in one patient model, highlighting a potential therapeutic approach. These data reveal shared pathophysiological mechanisms contributing to a microphthalmia phenotype.
Keywords: PAX6; Z-IETD-FMK; apoptosis; extracellular matrix; eye morphogenesis; hiPSC-derived optic vesicles; microphthalmia; ocular genetics; proliferation.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
Similar articles
-
Variant-specific disruption to notch signalling in PAX6 microphthalmia and aniridia patient-derived hiPSC optic cup-like organoids.Biochim Biophys Acta Mol Basis Dis. 2025 Aug;1871(6):167869. doi: 10.1016/j.bbadis.2025.167869. Epub 2025 Apr 23. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 40280197
-
The Role of FGF9 in the Production of Neural Retina and RPE in a Pluripotent Stem Cell Model of Early Human Retinal Development.Am J Ophthalmol. 2019 Oct;206:113-131. doi: 10.1016/j.ajo.2019.04.033. Epub 2019 May 10. Am J Ophthalmol. 2019. PMID: 31078532 Free PMC article.
-
Regulation of WNT Signaling by VSX2 During Optic Vesicle Patterning in Human Induced Pluripotent Stem Cells.Stem Cells. 2016 Nov;34(11):2625-2634. doi: 10.1002/stem.2414. Epub 2016 Jul 5. Stem Cells. 2016. PMID: 27301076 Free PMC article.
-
Ocular coloboma: Genetic variants reveal a dynamic model of eye development.Am J Med Genet C Semin Med Genet. 2020 Sep;184(3):590-610. doi: 10.1002/ajmg.c.31831. Epub 2020 Aug 27. Am J Med Genet C Semin Med Genet. 2020. PMID: 32852110 Review.
-
Conserved genetic pathways associated with microphthalmia, anophthalmia, and coloboma.Birth Defects Res C Embryo Today. 2015 Jun;105(2):96-113. doi: 10.1002/bdrc.21097. Epub 2015 Jun 3. Birth Defects Res C Embryo Today. 2015. PMID: 26046913 Free PMC article. Review.
Cited by
-
Genetic and environmental factors contributing to anophthalmia and microphthalmia: Current understanding and future directions.World J Clin Pediatr. 2025 Jun 9;14(2):101982. doi: 10.5409/wjcp.v14.i2.101982. eCollection 2025 Jun 9. World J Clin Pediatr. 2025. PMID: 40491727 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

