Recent advances and impending challenges for the radiopharmaceutical sciences in oncology
- PMID: 38821098
- PMCID: PMC11340123
- DOI: 10.1016/S1470-2045(24)00030-5
Recent advances and impending challenges for the radiopharmaceutical sciences in oncology
Abstract
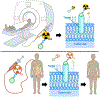
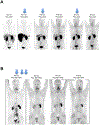
This paper is the first of a Series on theranostics that summarises the current landscape of the radiopharmaceutical sciences as they pertain to oncology. In this Series paper, we describe exciting developments in radiochemistry and the production of radionuclides, the development and translation of theranostics, and the application of artificial intelligence to our field. These developments are catalysing growth in the use of radiopharmaceuticals to the benefit of patients worldwide. We also highlight some of the key issues to be addressed in the coming years to realise the full potential of radiopharmaceuticals to treat cancer.
Copyright © 2024 Elsevier Ltd. All rights reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Declaration of interests SEL reports research support from Navidea Biopharmaceuticals, Fusion Pharmaceuticals, Cytosite Biopharma, Viewpoint Molecular Targeting, and Genzyme Corporation; has acted as an advisor for NorthStar Medical Radioisotopes and Trevarx Biomedical; and is supported by the Department of Energy as part of the Department of Energy University Isotope Network under grant DESC0021269. PJHS reports research support from Bristol Myers Squibb, Telix Pharmaceuticals, and Radionetics Oncology; has acted as an adviser to Synfast Consulting and Telix Pharmaceuticals; holds equity in Bristol Myers Squibb, Telix Pharmaceuticals, and Novartis; and is supported by National Institutes of Health R01 EB021155. AMS reports trial funding from EMD Serono, ITM, Telix Pharmaceuticals, AVID Radiopharmaceuticals, Fusion Pharmaceuticals, and Cyclotek; research funding from Medimmune, AVID Radiopharmaceuticals, Adalta, Antengene, Humanigen, Telix Pharmaceuticals, and Theramyc; is on the advisory boards of Imagion and ImmunOs; and is supported by Australian National Health and Medical Research Council grant number 1177837. ADW reports his role as the Editor in Chief of Nuclear Medicine and Biology. BMZ holds equity in Summit Biomedical Imaging. RPB is an advisor to 3B Pharmaceuticals (Berlin, Germany), ITM, Full Life Technologies, Sinotau, Jiangsu Huayi Technology, and Telix Pharmaceuticals. APK reports clinical trial funding from Novartis, Bayer, POINT, and Merck; and unpaid consulting for Novartis. JK reports an unrestricted grant from Janssen, and consulting fees from Telix and Novartis. JSL reports research support from Clarity Pharmaceuticals and Avid Radiopharmaceuticals; has acted as an adviser of Alpha-9 Theranostics, Clarity Pharmaceuticals, Earli, Evergreen Theragnostics, Inhibrix, Precirix, and Telix Pharmaceuticals; is a co-inventor on technologies licensed to Diaprost, Elucida Oncology, Theragnostics, CheMatech, Clarity Pharmaceuticals, and Samus Therapeutics; is the co-founder of pHLIP; holds equity in Summit Biomedical Imaging, Telix Pharmaceuticals, Clarity Pharmaceuticals, and Evergreen Theragnostics; and is supported by National Institutes of Health R35 CA232130. All other authors declare no competing interests.
Figures
References
-
- Sartor O, d. BJ, Chi KN, Fizazi K, Herrmann K, Rahbar K, Tagawa ST, Nordquist LT, Vaishampayan N, El-Haddad G, Park CH, Beer TM, Armour A, Pérez-Contreras WJ, DeSilvio M, Kpamegan E, Gericke G, Messmann RA, Morris MJ, Krause BJ, VISION Investigators. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med Sep 16, 1091–1103, doi:10.1056/NEJMoa2107322 (2021). - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

