Saliva antibody profiles are associated with reaction threshold and severity of peanut allergic reactions
- PMID: 38821318
- PMCID: PMC11380589
- DOI: 10.1016/j.jaci.2024.05.020
Saliva antibody profiles are associated with reaction threshold and severity of peanut allergic reactions
Abstract
Background: Reaction threshold and severity in food allergy are difficult to predict, and noninvasive predictors are lacking.
Objective: We sought to determine the relationships between pre-challenge levels of peanut (PN)-specific antibodies in saliva and reaction threshold, severity, and organ-specific symptoms during PN allergic reactions.
Methods: We measured PN-specific antibody levels in saliva collected from 127 children with suspected PN allergy before double-blind, placebo-controlled PN challenges in which reaction threshold, severity, and symptoms were rigorously characterized. Low threshold (LT) PN allergy was defined as reaction to <300 mg of PN protein cumulatively consumed. A consensus severity grading system was used to grade severity. We analyzed associations between antibody levels and reaction threshold, severity, and organ-specific symptoms.
Results: Among the 127 children, those with high pre-challenge saliva PN IgE had higher odds of LT PN allergy (odds ratio [OR] 3.9, 95% CI 1.6-9.5), while those with high saliva PN IgA:PN IgE ratio or PN IgG4:PN IgE ratio had lower odds of LT PN allergy (OR 0.3, 95% CI 0.1-0.8; OR 0.4, 95% CI 0.2-0.9). Children with high pre-challenge saliva PN IgG4 had lower odds of severe PN reactions (OR 0.4, 95% CI 0.2-0.9). Children with high saliva PN IgE had higher odds of respiratory symptoms (OR 8.0, 95% CI 2.2-26.8). Saliva PN IgE modestly correlated with serum PN IgE levels (Pearson r = 0.31, P = .0004). High and low saliva PN IgE levels further distinguished reaction threshold and severity in participants stratified by serum PN IgE, suggesting endotypes.
Conclusions: Saliva PN antibodies could aid in noninvasive risk stratification of PN allergy threshold, severity, and organ-specific symptoms.
Keywords: Peanut; allergy; antibody; endotype; food allergy; noninvasive; reaction; saliva; severity; symptom; threshold.
Copyright © 2024 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure statement This work was supported by National Institutes of Health grants R01AI147028 and U19AI136053. Disclosure of potential conflict of interest: S. Sicherer reports royalty payments from UpToDate and Johns Hopkins University Press; grants to his institution from the National Institute of Allergy and Infectious Diseases, Food Allergy Research & Education, and Pfizer; and personal fees from the American Academy of Allergy, Asthma & Immunology as Deputy Editor of the Journal of Allergy and Clinical Immunology: In Practice, outside of the submitted work. The remaining authors declare that they have no relevant conflicts of interest.
Figures
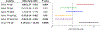
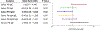
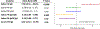

References
-
- Sicherer SH, Sampson HA. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141:41–58. - PubMed
-
- Jones SM, Burks AW. Food Allergy. N Engl J Med. 2017;377:1168–76. - PubMed
-
- Warren CM, Otto AK, Walkner MM, Gupta RS. Quality of Life Among Food Allergic Patients and Their Caregivers. Curr Allergy Asthma Rep. 2016;16:38. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

