Electrical stimulation of the ventral tegmental area restores consciousness from sevoflurane-, dexmedetomidine-, and fentanyl-induced unconsciousness in rats
- PMID: 38821397
- PMCID: PMC11212499
- DOI: 10.1016/j.brs.2024.05.012
Electrical stimulation of the ventral tegmental area restores consciousness from sevoflurane-, dexmedetomidine-, and fentanyl-induced unconsciousness in rats
Abstract
Background: Dopaminergic neurons in the ventral tegmental area (VTA) are crucially involved in regulating arousal, making them a potential target for reversing general anesthesia. Electrical deep brain stimulation (DBS) of the VTA restores consciousness in animals anesthetized with drugs that primarily enhance GABAA receptors. However, it is unknown if VTA DBS restores consciousness in animals anesthetized with drugs that target other receptors.
Objective: To evaluate the efficacy of VTA DBS in restoring consciousness after exposure to four anesthetics with distinct receptor targets.
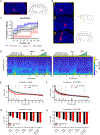
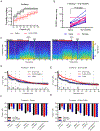
Methods: Sixteen adult Sprague-Dawley rats (8 female, 8 male) with bipolar electrodes implanted in the VTA were exposed to dexmedetomidine, fentanyl, ketamine, or sevoflurane to produce loss of righting, a proxy for unconsciousness. After receiving the dopamine D1 receptor antagonist, SCH-23390, or saline (vehicle), DBS was initiated at 30 μA and increased by 10 μA until reaching a maximum of 100 μA. The current that evoked behavioral arousal and restored righting was recorded for each anesthetic and compared across drug (saline/SCH-23390) condition. Electroencephalogram, heart rate and pulse oximetry were recorded continuously.
Results: VTA DBS restored righting after sevoflurane, dexmedetomidine, and fentanyl-induced unconsciousness, but not ketamine-induced unconsciousness. D1 receptor antagonism diminished the efficacy of VTA stimulation following sevoflurane and fentanyl, but not dexmedetomidine.
Conclusions: Electrical DBS of the VTA restores consciousness in animals anesthetized with mechanistically distinct drugs, excluding ketamine. The involvement of the D1 receptor in mediating this effect is anesthetic-specific.
Keywords: Dexmedetomidine; Dopamine D1 receptor; Fentanyl; General anesthesia; Ketamine; Ventral tegmental area.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Ken Solt reports financial support was provided by National Institutes of Health and the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Electrical stimulation of the ventral tegmental area induces reanimation from general anesthesia.Anesthesiology. 2014 Aug;121(2):311-9. doi: 10.1097/ALN.0000000000000117. Anesthesiology. 2014. PMID: 24398816 Free PMC article.
-
D-Amphetamine Rapidly Reverses Dexmedetomidine-Induced Unconsciousness in Rats.Front Pharmacol. 2021 May 18;12:668285. doi: 10.3389/fphar.2021.668285. eCollection 2021. Front Pharmacol. 2021. PMID: 34084141 Free PMC article.
-
Cellular stress and AMPK links metformin and diverse compounds with accelerated emergence from anesthesia and potential recovery from disorders of consciousness.Med Hypotheses. 2019 Mar;124:42-52. doi: 10.1016/j.mehy.2019.01.014. Epub 2019 Jan 22. Med Hypotheses. 2019. PMID: 30798915
-
Modulating anesthetic emergence with pathway-selective dopamine signaling.Curr Opin Anaesthesiol. 2023 Oct 1;36(5):468-475. doi: 10.1097/ACO.0000000000001293. Epub 2023 Jul 10. Curr Opin Anaesthesiol. 2023. PMID: 37552017 Free PMC article. Review.
-
Role of the ventral tegmental area in general anesthesia.Eur J Pharmacol. 2025 Jan 5;986:177145. doi: 10.1016/j.ejphar.2024.177145. Epub 2024 Nov 19. Eur J Pharmacol. 2025. PMID: 39566814 Review.
Cited by
-
High-Frequency Stimulation of the Ventral Tegmental Area Rescues Respiratory Failure.Res Sq [Preprint]. 2025 Jun 23:rs.3.rs-6740056. doi: 10.21203/rs.3.rs-6740056/v1. Res Sq. 2025. PMID: 40678236 Free PMC article. Preprint.
-
Dopaminergic modulation of propofol-induced activation in VLPO neurons: the role of D1 receptors in sleep-promoting neural circuits.Front Neurosci. 2025 Jan 8;18:1485873. doi: 10.3389/fnins.2024.1485873. eCollection 2024. Front Neurosci. 2025. PMID: 39844851 Free PMC article.
References
-
- Weiser TG, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008;372(9633):139–44. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

