Phase 2 study of pegylated liposomal doxorubicin plus cyclophosphamide, vincristine/vindesine, and prednisone in newly diagnosed PTCL: 8-year results
- PMID: 38821519
- PMCID: PMC11379645
- DOI: 10.1093/oncolo/oyae108
Phase 2 study of pegylated liposomal doxorubicin plus cyclophosphamide, vincristine/vindesine, and prednisone in newly diagnosed PTCL: 8-year results
Abstract
Background: Pegylated liposomal doxorubicin (PLD) is a liposome-encapsulated form of doxorubicin with equivalent efficacy and less cardiotoxicity. This phase 2 study evaluated the efficacy and safety of the PLD-containing CHOP regimen in newly diagnosed patients with aggressive peripheral T-cell lymphomas (PTCL).
Methods: Patients received PLD, cyclophosphamide, vincristine/vindesine, plus prednisone every 3 weeks for up to 6 cycles. The primary endpoint was the objective response rate at the end of treatment (EOT).
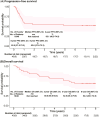
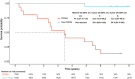
Results: From September 2015 to January 2017, 40 patients were treated. At the EOT, objective response was achieved by 82.5% of patients, with 62.5% complete response. As of the cutoff date (September 26, 2023), median progression-free survival (mPFS) and overall survival (mOS) were not reached (NR). The 2-year, 5-year, and 8-year PFS rates were 55.1%, 52.0%, and 52.0%. OS rate was 80.0% at 2 years, 62.5% at 5 years, and 54.3% at 8 years. Patients with progression of disease within 24 months (POD24) had worse prognosis than those without POD24, regarding mOS (41.2 months vs NR), 5-year OS (33.3% vs 94.4%), and 8-year OS (13.3% vs 94.4%). Common grade 3-4 adverse events were neutropenia (87.5%), leukopenia (80.0%), anemia (17.5%), and pneumonitis (17.5%).
Conclusion: This combination had long-term benefits and manageable tolerability, particularly with less cardiotoxicity, for aggressive PTCL, which might provide a favorable benefit-risk balance.
Clinicaltrials.gov identifier: Chinese Clinical Trial Registry, ChiCTR2100054588; IRB Approved: Ethics committee of Fudan University Shanghai Cancer Center (Date 2015.8.31/No. 1508151-13.
Keywords: chemotherapy; first-line treatment; pegylated liposomal doxorubicin; peripheral T-cell lymphomas.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures

Similar articles
-
Pegylated liposomal doxorubicin replacing conventional doxorubicin in standard R-CHOP chemotherapy for elderly patients with diffuse large B-cell lymphoma: an open label, single arm, phase II trial.Clin Lymphoma Myeloma Leuk. 2015 Mar;15(3):152-8. doi: 10.1016/j.clml.2014.09.001. Epub 2014 Sep 28. Clin Lymphoma Myeloma Leuk. 2015. PMID: 25445468 Free PMC article. Clinical Trial.
-
Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study.J Clin Oncol. 2014 Oct 1;32(28):3137-43. doi: 10.1200/JCO.2013.54.2456. Epub 2014 Aug 18. J Clin Oncol. 2014. PMID: 25135998 Free PMC article. Clinical Trial.
-
Pharmacodynamic and pharmacokinetic study of pegylated liposomal doxorubicin combination (CCOP) chemotherapy in patients with peripheral T-cell lymphomas.Acta Pharmacol Sin. 2011 Mar;32(3):408-14. doi: 10.1038/aps.2010.217. Acta Pharmacol Sin. 2011. PMID: 21372831 Free PMC article.
-
Cardiovascular adverse events in patients with non-Hodgkin lymphoma treated with first-line cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP with rituximab (R-CHOP): a systematic review and meta-analysis.Lancet Haematol. 2020 Apr;7(4):e295-e308. doi: 10.1016/S2352-3026(20)30031-4. Epub 2020 Mar 2. Lancet Haematol. 2020. PMID: 32135128
-
The clinical and economic burden of peripheral T-cell lymphoma: a systematic literature review.Future Oncol. 2022 Feb;18(4):519-535. doi: 10.2217/fon-2021-1032. Epub 2021 Dec 1. Future Oncol. 2022. PMID: 34851173
References
-
- Plana JC, Galderisi M, Barac A, et al.. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imag. 2014;15(10):1063-1093. 10.1093/ehjci/jeu192 - DOI - PMC - PubMed
-
- Wang J, Gao L, Qiu H, et al.. Comparative study on the efficacy of hyper CVAD/MA regimen and CHOP or CHOP like regimen in the treatment of primary peripheral T cell lymphoma. Zhonghua Xue Ye Xue Za Zhi. 2014;35(10):897-900. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

