Cytokine-armed oncolytic herpes simplex viruses: a game-changer in cancer immunotherapy?
- PMID: 38821716
- PMCID: PMC11149157
- DOI: 10.1136/jitc-2023-008025
Cytokine-armed oncolytic herpes simplex viruses: a game-changer in cancer immunotherapy?
Abstract
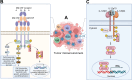
Cytokines are small proteins that regulate the growth and functional activity of immune cells, and several have been approved for cancer therapy. Oncolytic viruses are agents that mediate antitumor activity by directly killing tumor cells and inducing immune responses. Talimogene laherparepvec is an oncolytic herpes simplex virus type 1 (oHSV), approved for the treatment of recurrent melanoma, and the virus encodes the human cytokine, granulocyte-macrophage colony-stimulating factor (GM-CSF). A significant advantage of oncolytic viruses is the ability to deliver therapeutic payloads to the tumor site that can help drive antitumor immunity. While cytokines are especially interesting as payloads, the optimal cytokine(s) used in oncolytic viruses remains controversial. In this review, we highlight preliminary data with several cytokines and chemokines, including GM-CSF, interleukin 12, FMS-like tyrosine kinase 3 ligand, tumor necrosis factor α, interleukin 2, interleukin 15, interleukin 18, chemokine (C-C motif) ligand 2, chemokine (C-C motif) ligand 5, chemokine (C-X-C motif) ligand 4, or their combinations, and show how these payloads can further enhance the antitumor immunity of oHSV. A better understanding of cytokine delivery by oHSV can help improve clinical benefit from oncolytic virus immunotherapy in patients with cancer.
Keywords: Cytokine; Immune modulatory; Oncolytic virus.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SDR is a co-inventor on patents relating to oncolytic herpes simplex viruses, owned, and managed by Georgetown University and Massachusetts General Hospital, which have received royalties from Amgen and Acti\Vec Inc., and acted as a consultant and received honoraria from Replimune, Cellinta, and Greenfire Bio, and honoraria and equity from EG 427. HLK is an employee of Ankyra Therapeutics and has received honoraria for participating on advisory boards for Castle Biosciences, Midatech Pharma, Marengo Therapeutics, and Virogin. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Oncolytic herpes simplex virus and immunotherapy.BMC Immunol. 2018 Dec 18;19(1):40. doi: 10.1186/s12865-018-0281-9. BMC Immunol. 2018. PMID: 30563466 Free PMC article. Review.
-
Talimogene laherparepvec (T-VEC) as cancer immunotherapy.Drugs Today (Barc). 2015 Sep;51(9):549-58. doi: 10.1358/dot.2015.51.9.2383044. Drugs Today (Barc). 2015. PMID: 26488034 Review.
-
Potentiating Oncolytic Virus-Induced Immune-Mediated Tumor Cell Killing Using Histone Deacetylase Inhibition.Mol Ther. 2019 Jun 5;27(6):1139-1152. doi: 10.1016/j.ymthe.2019.04.008. Epub 2019 Apr 14. Mol Ther. 2019. PMID: 31053413 Free PMC article.
-
Talimogene laherparepvec: First in class oncolytic virotherapy.Hum Vaccin Immunother. 2018 Apr 3;14(4):839-846. doi: 10.1080/21645515.2017.1412896. Epub 2018 Feb 22. Hum Vaccin Immunother. 2018. PMID: 29420123 Free PMC article. Review.
-
Development of a new fusion-enhanced oncolytic immunotherapy platform based on herpes simplex virus type 1.J Immunother Cancer. 2019 Aug 10;7(1):214. doi: 10.1186/s40425-019-0682-1. J Immunother Cancer. 2019. PMID: 31399043 Free PMC article.
Cited by
-
Gnostic and agnostic immunotherapy by tropism-retargeted herpes simplex virus without direct tumor treatment.J Immunother Cancer. 2025 Jul 22;13(7):e011812. doi: 10.1136/jitc-2025-011812. J Immunother Cancer. 2025. PMID: 40701653 Free PMC article.
-
Enhancing cancer therapy: the integration of oncolytic virus therapy with diverse treatments.Cancer Cell Int. 2024 Jul 11;24(1):242. doi: 10.1186/s12935-024-03424-z. Cancer Cell Int. 2024. PMID: 38992667 Free PMC article. Review.
-
Advances in the Drug Development and Quality Evaluation Principles of Oncolytic Herpes Simplex Virus.Viruses. 2025 Apr 18;17(4):581. doi: 10.3390/v17040581. Viruses. 2025. PMID: 40285023 Free PMC article. Review.
-
Combining local cytokine delivery and systemic immunization with recombinant artLCMV boosts antitumor efficacy in several preclinical tumor models.Oncoimmunology. 2025 Dec;14(1):2514040. doi: 10.1080/2162402X.2025.2514040. Epub 2025 Jun 10. Oncoimmunology. 2025. PMID: 40492380 Free PMC article.
-
Simultaneous Expression of Different Therapeutic Genes by Infection with Multiple Oncolytic HSV-1 Vectors.Biomedicines. 2024 Jul 16;12(7):1577. doi: 10.3390/biomedicines12071577. Biomedicines. 2024. PMID: 39062150 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
