Mechanistic study of the stabilization of dentin-bonded restorative interfaces via collagen reinforcement by multi-acrylamides
- PMID: 38821837
- PMCID: PMC11260233
- DOI: 10.1016/j.dental.2024.05.027
Mechanistic study of the stabilization of dentin-bonded restorative interfaces via collagen reinforcement by multi-acrylamides
Abstract
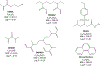
Hydrolytically and enzymatically-stable multi-acrylamides have been proposed to increase the long-term durability of dental adhesive interfaces as alternatives to methacrylates. The aim of this study was to investigate the mechanical and biochemical properties of experimental adhesives containing multi-functional acrylamides concerning collagen reinforcement and metalloproteinases (MMP) activity. Multi-functional acrylamides, TMAAEA (Tris[(2-methylaminoacryl) ethylamine) and DEBAAP (N,N-Diethyl-1,3-bis(acrylamido) propane), along with the commercially available DMAM (N,N-dimethylacrylamide) (monofunctional acrylamide) and HEMA (2-Hydroxyethyl methacrylate) (monofunctional methacrylate - control) were tested for stability against enzymatic hydrolysis by cholesterol esterase/pseudocholinesterase (PC/PCE) solutions for up to 30 days. Collagen-derived substrate and gelatin zymography were performed to examine the effect of the compounds on the biological activity of human recombinant and dentin-extracted gelatinases MMP-2 and MMP-9. In situ zymography was carried out by fluorescent collagen degradation combined with confocal microscopy analysis. Hydroxyproline content was measured in collagen derived from dentin extracts though reaction with Ehrlich's reagent p-dimethylaminobenzaldehyde (DMAB), generating a stable chromophore measured at 550 nm. Storage shear modulus of demineralized dentin discs treated with the tested compounds was measured by oscillatory rheometry, in order to investigate potential collagen reinforcement. FT-IR was performed to determine qualitative differences in collagen based on observed changes in amide bands. The results were analyzed by ANOVA/Tukey's test (α = 0.05). Multi-acrylamides survived 30 days of incubation in cholinesterase/pseudo-cholinesterase (PC/PCE) solutions, while HEMA showed approximately 70 % overall degradation. Incubation with multi-acrylamides reduced collagen degradation as evidenced by the reduced hydroxyproline levels and by the 30 % increase inshear storage modulus. Biochemical and zymography assays showed no noticeable inhibition of recombinant and extracted MMPs enzymatic activity. The infra-red spectroscopy results for multi-functional acrylamides treated samples demonstrated shifts of the amide II bonds and marked increase in intensity of the bands 1200 cm-1, which may indicate partial collagen denaturation and some degree of cross-linking of the compounds with collagen, respectively. The multi-acrylamides exhibited not only comparable mechanical properties but also demonstrated significantly enhanced biochemical stability when compared to the widely used methacrylate control. Clinical relevance: These findings highlight the potential of multi-acrylamides to increase the bonding stability to tissues and, ultimately, contribute to the longevity of dental restorations.
Keywords: Acrylamide; Adhesive interface; Dental restorations; In-situ zymography; MMP-2 MMP-9; Methacrylate.
Copyright © 2024 Elsevier Inc. All rights reserved.
Figures
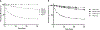
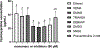


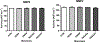
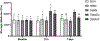
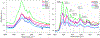
Similar articles
-
Effects of a Hydrophilic Collagen-Reactive Monomer on Dentin Bond Durability.J Dent Res. 2025 Jul;104(7):734-742. doi: 10.1177/00220345251316434. Epub 2025 Mar 14. J Dent Res. 2025. PMID: 40087615
-
Dental pulp enhances dentin bonding durability: Evidence from a rat model.J Dent. 2025 Sep;160:105925. doi: 10.1016/j.jdent.2025.105925. Epub 2025 Jun 20. J Dent. 2025. PMID: 40545231
-
Is the presence of 10-MDP associated to higher bonding performance for self-etching adhesive systems? A meta-analysis of in vitro studies.Dent Mater. 2021 Oct;37(10):1463-1485. doi: 10.1016/j.dental.2021.08.014. Epub 2021 Aug 26. Dent Mater. 2021. PMID: 34456050
-
Prodelphinidins enhance dentin matrix properties and promote adhesion to methacrylate resin.Dent Mater. 2024 Aug;40(8):1164-1170. doi: 10.1016/j.dental.2024.05.024. Epub 2024 Jun 12. Dent Mater. 2024. PMID: 38871526 Free PMC article.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
Cited by
-
Preservation Strategies for Interfacial Integrity in Restorative Dentistry: A Non-Comprehensive Literature Review.J Funct Biomater. 2025 Jan 26;16(2):42. doi: 10.3390/jfb16020042. J Funct Biomater. 2025. PMID: 39997576 Free PMC article. Review.
-
Zymography as a Tool for Exploring Protease Activity in Dental Research.Methods Mol Biol. 2025;2918:89-105. doi: 10.1007/978-1-0716-4482-9_8. Methods Mol Biol. 2025. PMID: 40261616
-
Bond preservation in caries-affected dentin restored with acrylamide-based adhesives.Dent Mater. 2025 Jul;41(7):788-797. doi: 10.1016/j.dental.2025.04.008. Epub 2025 May 15. Dent Mater. 2025. PMID: 40379529
References
-
- Frassetto, Breschi L, Turco G, Marchesi G, Di Lenarda R, Tay FR, Pashley DH, Cadenaro M, Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability—A literature review, Dental Materials 32(2) (2016) e41–e53. - PubMed
-
- Demarco FF, Corrêa MB, Cenci MS, Moraes RR, Opdam NJ, Longevity of posterior composite restorations: not only a matter of materials, Dent Mater 28(1) (2012) 87–101. - PubMed
-
- Chersoni S, Suppa P, Grandini S, Goracci C, Monticelli F, Yiu C, Huang C, Prati C, Breschi L, Ferrari M, Pashley DH, Tay FR, In vivo and in vitro permeability of one-step self-etch adhesives, J Dent Res 83(6) (2004) 459–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

