The neural basis of resting-state fMRI functional connectivity in fronto-limbic circuits revealed by chemogenetic manipulation
- PMID: 38821963
- PMCID: PMC11143237
- DOI: 10.1038/s41467-024-49140-0
The neural basis of resting-state fMRI functional connectivity in fronto-limbic circuits revealed by chemogenetic manipulation
Abstract
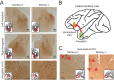
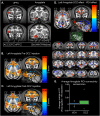
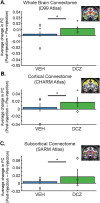
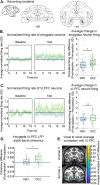
Measures of fMRI resting-state functional connectivity (rs-FC) are an essential tool for basic and clinical investigations of fronto-limbic circuits. Understanding the relationship between rs-FC and the underlying patterns of neural activity in these circuits is therefore vital. Here we introduced inhibitory designer receptors exclusively activated by designer drugs (DREADDs) into the amygdala of two male macaques. We evaluated the causal effect of activating the DREADD receptors on rs-FC and neural activity within circuits connecting amygdala and frontal cortex. Activating the inhibitory DREADD increased rs-FC between amygdala and ventrolateral prefrontal cortex. Neurophysiological recordings revealed that the DREADD-induced increase in fMRI rs-FC was associated with increased local field potential coherency in the alpha band (6.5-14.5 Hz) between amygdala and ventrolateral prefrontal cortex. Thus, our multi-modal approach reveals the specific signature of neuronal activity that underlies rs-FC in fronto-limbic circuits.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Update of
-
The neural basis of resting-state fMRI functional connectivity in fronto-limbic circuits revealed by chemogenetic manipulation.bioRxiv [Preprint]. 2023 Sep 15:2023.06.21.545778. doi: 10.1101/2023.06.21.545778. bioRxiv. 2023. Update in: Nat Commun. 2024 May 31;15(1):4669. doi: 10.1038/s41467-024-49140-0. PMID: 37745436 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- R01 MH111439/MH/NIMH NIH HHS/United States
- R01MH110822/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- R01MH111439/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- RF1 MH117040/MH/NIMH NIH HHS/United States
- RF1MH117040/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

