Bio-synthesis, purification and structural analysis of Cyclosporine-A produced by Tolypocladium inflatum with valorization of agro-industrial wastes
- PMID: 38822034
- PMCID: PMC11143273
- DOI: 10.1038/s41598-024-63110-y
Bio-synthesis, purification and structural analysis of Cyclosporine-A produced by Tolypocladium inflatum with valorization of agro-industrial wastes
Abstract
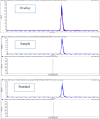
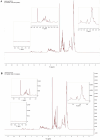
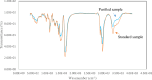
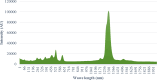
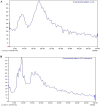
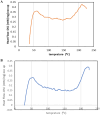
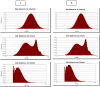
Cyclosporine A (CyA) holds significant importance as a strategic immunosuppressive drug for organ transplant patients. In this study, we aimed to produce pure and cost-effective Cyclosporine A (CyA) by fermenting a culture medium containing dairy sludge, using Tolypocladium inflatum PTCC 5253. Following the fermentation stage, ethyl acetate extraction and fast protein liquid chromatography were employed for sample purification. The initial evaluation of the effectiveness of CyA obtained from these processes was performed through bioassay, wherein the antimicrobial clear zone diameter was found to be larger compared to the sample obtained from the fermentation culture. The concentration of CyA was determined using high-performance liquid chromatography, yielding values of 334 mg/L, 456 mg/L, and 578 mg/L for the fermented, extracted, and purified samples, respectively. Further analysis utilizing liquid chromatography tandem mass spectrometry (LC/MS/MS) confirmed a purity of 91.9% and proper agreement with the standard sample based on the ion intensity of Z/m 1205. To validate the structure of CyA, nuclear magnetic resonance spectroscopy, Fourier-transform infrared (FT-IR), and Raman spectroscopy were employed. X-ray diffraction and differential scanning calorimetry analyses demonstrated that the purified CyA exhibited a crystal structure similar to the standard sample, characterized by two broad peaks at 2θ = 9° and 20°, and comparable glass transition temperatures (57-68 °C for the purified sample; 53-64 °C for the standard sample). Dynamic light scattering analysis confirmed a uniform particle size distribution in both the purified and standard samples. The zeta potentials of the purified and standard samples were determined to be - 25.8 ± 0.16 and - 23.63 ± 0.12 mV, respectively. Our results demonstrate that dairy sludge can serve as a suitable culture medium for the production of (CyA).
Keywords: Tolypocladium inflatum; Chemical structure; Cyclosporine; Purification.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Effect of temperature on the extraction kinetics and diffusivity of Cyclosporin A in the fungus Tolypocladium inflatum.Biotechnol Bioeng. 2007 Apr 1;96(5):945-55. doi: 10.1002/bit.21198. Biotechnol Bioeng. 2007. PMID: 17009335
-
A novel medium for the enhanced production of cyclosporin A by Tolypocladium inflatum MTCC 557 using solid state fermentation.J Microbiol Biotechnol. 2009 May;19(5):462-7. doi: 10.4014/jmb.0805.324. J Microbiol Biotechnol. 2009. PMID: 19494693
-
Effect of solvent concentration on the extraction kinetics and diffusivity of Cyclosporin A in the fungus Tolypocladium inflatum.Biotechnol Bioeng. 2007 Jan 1;96(1):67-79. doi: 10.1002/bit.21180. Biotechnol Bioeng. 2007. PMID: 16948167
-
Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: analytical techniques and challenges.J Pharm Biomed Anal. 2014 Jan;87:176-90. doi: 10.1016/j.jpba.2013.04.037. Epub 2013 May 6. J Pharm Biomed Anal. 2014. PMID: 23721687 Review.
-
Cyclosporin A--a review on fermentative production, downstream processing and pharmacological applications.Biotechnol Adv. 2011 Jul-Aug;29(4):418-35. doi: 10.1016/j.biotechadv.2011.03.004. Epub 2011 Apr 5. Biotechnol Adv. 2011. PMID: 21447377 Review.
Cited by
-
A Comprehensive Review of the Diversity of Fungal Secondary Metabolites and Their Emerging Applications in Healthcare and Environment.Mycobiology. 2024 Dec 3;52(6):335-387. doi: 10.1080/12298093.2024.2416736. eCollection 2024. Mycobiology. 2024. PMID: 39845176 Free PMC article. Review.
-
Application of fatty acid-based eutectic mixture as a phase change material in microencapsulation of drugs: preparation, characterization and release behavior.BMC Chem. 2025 Feb 17;19(1):42. doi: 10.1186/s13065-025-01406-4. BMC Chem. 2025. PMID: 39962482 Free PMC article.
-
Regulation and induction of fungal secondary metabolites: a comprehensive review.Arch Microbiol. 2025 Jul 1;207(8):189. doi: 10.1007/s00203-025-04386-0. Arch Microbiol. 2025. PMID: 40590991 Review.
References
-
- Ghazanfari N, Fallah S, Vasiee A, Yazdi FT. Optimization of fermentation culture medium containing food waste for l-glutamate production using native lactic acid bacteria and comparison with industrial strain. LWT. 2023;184:114871. doi: 10.1016/j.lwt.2023.114871. - DOI
-
- Rao HY, Kamalraj S, Jayabaskaran C. Fascinating fungal endophytes associated with medicinal plants: Recent advances and beneficial applications. In: Kumar A, Singh VK, editors. Microbial Endophytes. Elsevier; 2020. pp. 263–289.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

