Predicting mortality after transcatheter aortic valve replacement using preprocedural CT
- PMID: 38822074
- PMCID: PMC11143216
- DOI: 10.1038/s41598-024-63022-x
Predicting mortality after transcatheter aortic valve replacement using preprocedural CT
Erratum in
-
Author Correction: Predicting mortality after transcatheter aortic valve replacement using preprocedural CT.Sci Rep. 2025 Mar 26;15(1):10460. doi: 10.1038/s41598-025-94409-z. Sci Rep. 2025. PMID: 40140477 Free PMC article. No abstract available.
Abstract
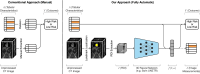
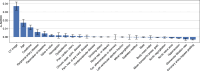
Transcatheter aortic valve replacement (TAVR) is a widely used intervention for patients with severe aortic stenosis. Identifying high-risk patients is crucial due to potential postprocedural complications. Currently, this involves manual clinical assessment and time-consuming radiological assessment of preprocedural computed tomography (CT) images by an expert radiologist. In this study, we introduce a probabilistic model that predicts post-TAVR mortality automatically using unprocessed, preprocedural CT and 25 baseline patient characteristics. The model utilizes CT volumes by automatically localizing and extracting a region of interest around the aortic root and ascending aorta. It then extracts task-specific features with a 3D deep neural network and integrates them with patient characteristics to perform outcome prediction. As missing measurements or even missing CT images are common in TAVR planning, the proposed model is designed with a probabilistic structure to allow for marginalization over such missing information. Our model demonstrates an AUROC of 0.725 for predicting all-cause mortality during postprocedure follow-up on a cohort of 1449 TAVR patients. This performance is on par with what can be achieved with lengthy radiological assessments performed by experts. Thus, these findings underscore the potential of the proposed model in automatically analyzing CT volumes and integrating them with patient characteristics for predicting mortality after TAVR.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Utility of Functional and Volumetric Left Atrial Parameters Derived From Preprocedural Cardiac CTA in Predicting Mortality After Transcatheter Aortic Valve Replacement.AJR Am J Roentgenol. 2022 Mar;218(3):444-452. doi: 10.2214/AJR.21.26775. Epub 2021 Oct 13. AJR Am J Roentgenol. 2022. PMID: 34643107
-
Two-year survival of patients screened for transcatheter aortic valve replacement with potentially malignant incidental findings in initial body computed tomography.Eur Heart J Cardiovasc Imaging. 2015 Jul;16(7):731-7. doi: 10.1093/ehjci/jev055. Epub 2015 Mar 10. Eur Heart J Cardiovasc Imaging. 2015. PMID: 25759083 Free PMC article.
-
Preprocedure CT Findings of Right Heart Failure as a Predictor of Mortality After Transcatheter Aortic Valve Replacement.AJR Am J Roentgenol. 2021 Jan;216(1):57-65. doi: 10.2214/AJR.20.22894. Epub 2020 Nov 10. AJR Am J Roentgenol. 2021. PMID: 33170737
-
Usefulness of skeletal muscle area detected by computed tomography to predict mortality in patients undergoing transcatheter aortic valve replacement: a meta-analysis study.Int J Cardiovasc Imaging. 2019 Jun;35(6):1141-1147. doi: 10.1007/s10554-019-01582-0. Epub 2019 Mar 26. Int J Cardiovasc Imaging. 2019. PMID: 30915667
-
Transcatheter Aortic Valve Replacement: Imaging Techniques for Aortic Root Sizing.J Thorac Imaging. 2015 Nov;30(6):349-58. doi: 10.1097/RTI.0000000000000167. J Thorac Imaging. 2015. PMID: 26164166 Review.
Cited by
-
The Current Landscape of Artificial Intelligence in Imaging for Transcatheter Aortic Valve Replacement.Curr Radiol Rep. 2024;12(11-12):113-120. doi: 10.1007/s40134-024-00431-w. Epub 2024 Oct 10. Curr Radiol Rep. 2024. PMID: 39483792 Free PMC article.
-
Artificial Intelligence in Risk Stratification and Outcome Prediction for Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis.J Pers Med. 2025 Jul 11;15(7):302. doi: 10.3390/jpm15070302. J Pers Med. 2025. PMID: 40710419 Free PMC article. Review.
References
-
- Vahanian, A. et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease: Developed by the task force for the management of valvular heart disease of the European society of cardiology (ESC) and the European association for cardio-thoracic surgery (EACTS). Eur. Heart J.43, 561–632 (2022). - PubMed
-
- Reardon, M. J. et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N. Engl. J. Med.376, 1321–1331 (2017). - PubMed
-
- Eberhard, M. et al. Incremental prognostic value of coronary artery calcium score for predicting all-cause mortality after transcatheter aortic valve replacement. Radiology301, 105–112 (2021). - PubMed
-
- Kuzo, N. et al. Outcome of patients with severe aortic stenosis and normal coronary arteries undergoing transcatheter aortic valve implantation. Am. J. Cardiol.143, 89–96 (2021). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

