AGR2-mediated unconventional secretion of 14-3-3ε and α-actinin-4, responsive to ER stress and autophagy, drives chemotaxis in canine mammary tumor cells
- PMID: 38822246
- PMCID: PMC11140979
- DOI: 10.1186/s11658-024-00601-w
AGR2-mediated unconventional secretion of 14-3-3ε and α-actinin-4, responsive to ER stress and autophagy, drives chemotaxis in canine mammary tumor cells
Abstract
Background: Canine mammary tumors (CMTs) in intact female dogs provide a natural model for investigating metastatic human cancers. Our prior research identified elevated expression of Anterior Gradient 2 (AGR2), a protein disulfide isomerase (PDI) primarily found in the endoplasmic reticulum (ER), in CMT tissues, highly associated with CMT progression. We further demonstrated that increased AGR2 expression actively influences the extracellular microenvironment, promoting chemotaxis in CMT cells. Unraveling the underlying mechanisms is crucial for assessing the potential of therapeutically targeting AGR2 as a strategy to inhibit a pro-metastatic microenvironment and impede tumor metastasis.
Methods: To identify the AGR2-modulated secretome, we employed proteomics analysis of the conditioned media (CM) from two CMT cell lines ectopically expressing AGR2, compared with corresponding vector-expressing controls. AGR2-regulated release of 14-3-3ε (gene: YWHAE) and α-actinin 4 (gene: ACTN4) was validated through ectopic expression, knockdown, and knockout of the AGR2 gene in CMT cells. Extracellular vesicles derived from CMT cells were isolated using either differential ultracentrifugation or size exclusion chromatography. The roles of 14-3-3ε and α-actinin 4 in the chemotaxis driven by the AGR2-modulated CM were investigated through gene knockdown, antibody-mediated interference, and recombinant protein supplement. Furthermore, the clinical relevance of the release of 14-3-3ε and α-actinin 4 was assessed using CMT tissue-immersed saline and sera from CMT-afflicted dogs.
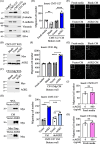
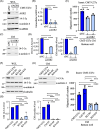
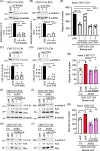
Results: Proteomics analysis of the AGR2-modulated secretome revealed increased abundance in 14-3-3ε and α-actinin 4. Ectopic expression of AGR2 significantly increased the release of 14-3-3ε and α-actinin 4 in the CM. Conversely, knockdown or knockout of AGR2 expression remarkably reduced their release. Silencing 14-3-3ε or α-actinin 4 expression diminished the chemotaxis driven by AGR2-modulated CM. Furthermore, AGR2 controls the release of 14-3-3ε and α-actinin 4 primarily via non-vesicular routes, responding to the endoplasmic reticulum (ER) stress and autophagy activation. Knockout of AGR2 resulted in increased α-actinin 4 accumulation and impaired 14-3-3ε translocation in autophagosomes. Depletion of extracellular 14-3-3ε or α-actinin 4 reduced the chemotaxis driven by AGR2-modulated CM, whereas supplement with recombinant 14-3-3ε in the CM enhanced the CM-driven chemotaxis. Notably, elevated levels of 14-3-3ε or α-actinin 4 were observed in CMT tissue-immersed saline compared with paired non-tumor samples and in the sera of CMT dogs compared with healthy dogs.
Conclusion: This study elucidates AGR2's pivotal role in orchestrating unconventional secretion of 14-3-3ε and α-actinin 4 from CMT cells, thereby contributing to paracrine-mediated chemotaxis. The insight into the intricate interplay between AGR2-involved ER stress, autophagy, and unconventional secretion provides a foundation for refining strategies aimed at impeding metastasis in both canine mammary tumors and potentially human cancers.
Keywords: 14-3-3 Epsilon (YWHAE); Alpha-actinin 4 (ACTN4); Anterior gradient 2 (AGR2); Canine mammary tumor (CMT); Chemotaxis; Microenvironment; Proteomics; Unconventional protein secretion.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







Similar articles
-
Role of pro-oncogenic protein disulfide isomerase (PDI) family member anterior gradient 2 (AGR2) in the control of endoplasmic reticulum homeostasis.J Biol Chem. 2011 Dec 30;286(52):44855-68. doi: 10.1074/jbc.M111.275529. Epub 2011 Oct 24. J Biol Chem. 2011. PMID: 22025610 Free PMC article.
-
Extracellular AGR2 triggers lung tumour cell proliferation through repression of p21CIP1.Biochim Biophys Acta Mol Cell Res. 2021 Mar;1868(3):118920. doi: 10.1016/j.bbamcr.2020.118920. Epub 2020 Dec 2. Biochim Biophys Acta Mol Cell Res. 2021. PMID: 33278424
-
Control of anterior GRadient 2 (AGR2) dimerization links endoplasmic reticulum proteostasis to inflammation.EMBO Mol Med. 2019 Jun;11(6):e10120. doi: 10.15252/emmm.201810120. EMBO Mol Med. 2019. PMID: 31040128 Free PMC article.
-
The estrogen-regulated anterior gradient 2 (AGR2) protein in breast cancer: a potential drug target and biomarker.Breast Cancer Res. 2013 Apr 24;15(2):204. doi: 10.1186/bcr3408. Breast Cancer Res. 2013. PMID: 23635006 Free PMC article. Review.
-
AGR2: The Covert Driver and New Dawn of Hepatobiliary and Pancreatic Cancer Treatment.Biomolecules. 2024 Jun 23;14(7):743. doi: 10.3390/biom14070743. Biomolecules. 2024. PMID: 39062458 Free PMC article. Review.
Cited by
-
Multiplexed immunoassay for a serum autoantibody biomarker panel in diagnostic and prognostic prediction of canine mammary tumors.Vet Q. 2025 Dec;45(1):1-12. doi: 10.1080/01652176.2024.2435978. Epub 2024 Dec 6. Vet Q. 2025. PMID: 39639821 Free PMC article.
-
Neonatal hypoxia leads to impaired intestinal function and changes in the composition and metabolism of its microbiota.Sci Rep. 2025 May 1;15(1):15285. doi: 10.1038/s41598-025-00041-2. Sci Rep. 2025. PMID: 40312410 Free PMC article.
-
Human cytomegalovirus UL82 promotes cell cycle progression of colorectal cancer by upregulating AGR2.Commun Biol. 2025 Feb 17;8(1):251. doi: 10.1038/s42003-025-07674-z. Commun Biol. 2025. PMID: 39962326 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

