Therapeutic role of interferon-γ in experimental autoimmune encephalomyelitis is mediated through a tolerogenic subset of splenic CD11b+ myeloid cells
- PMID: 38822334
- PMCID: PMC11143617
- DOI: 10.1186/s12974-024-03126-3
Therapeutic role of interferon-γ in experimental autoimmune encephalomyelitis is mediated through a tolerogenic subset of splenic CD11b+ myeloid cells
Abstract
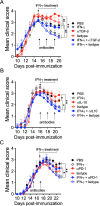
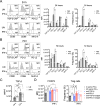
Cumulative evidence has established that Interferon (IFN)-γ has both pathogenic and protective roles in Multiple Sclerosis and the animal model, Experimental Autoimmune Encephalomyelitis (EAE). However, the underlying mechanisms to the beneficial effects of IFN-γ are not well understood. In this study, we found that IFN-γ exerts therapeutic effects on chronic, relapsing-remitting, and chronic progressive EAE models. The frequency of regulatory T (Treg) cells in spinal cords from chronic EAE mice treated with IFN-γ was significantly increased with no effect on Th1 and Th17 cells. Consistently, depletion of FOXP3-expressing cells blocked the protective effects of IFN-γ, indicating that the therapeutic effect of IFN-γ depends on the presence of Treg cells. However, IFN-γ did not trigger direct in vitro differentiation of Treg cells. In vivo administration of blocking antibodies against either interleukin (IL)-10, transforming growth factor (TGF)-β or program death (PD)-1, revealed that the protective effects of IFN-γ in EAE were also dependent on TGF-β and PD-1, but not on IL-10, suggesting that IFN-γ might have an indirect role on Treg cells acting through antigen-presenting cells. Indeed, IFN-γ treatment increased the frequency of a subset of splenic CD11b+ myeloid cells expressing TGF-β-Latency Associated Peptide (LAP) and program death ligand 1 (PD-L1) in a signal transducer and activator of transcription (STAT)-1-dependent manner. Furthermore, splenic CD11b+ cells from EAE mice preconditioned in vitro with IFN-γ and myelin oligodendrocyte glycoprotein (MOG) peptide exhibited a tolerogenic phenotype with the capability to induce conversion of naïve CD4+ T cells mediated by secretion of TGF-β. Remarkably, adoptive transfer of splenic CD11b+ cells from IFN-γ-treated EAE mice into untreated recipient mice ameliorated clinical symptoms of EAE and limited central nervous system infiltration of mononuclear cells and effector helper T cells. These results reveal a novel cellular and molecular mechanism whereby IFN-γ promotes beneficial effects in EAE by endowing splenic CD11b+ myeloid cells with tolerogenic and therapeutic activities.
Keywords: CD11b+ cells; Experimental autoimmune encephalomyelitis; Interferon-γ; Multiple sclerosis; Regulatory T cells; TGF-β.
© 2024. The Author(s).
Conflict of interest statement
SDM is a co-founder, paid consultant, scientific advisory board member, and grantee of COUR Pharmaceuticals. SDM is also a consultant for NextCure and Takeda Pharmaceuticals. The other authors have declared that no conflicts of interest exist.
Figures





Similar articles
-
Novel CD8+ Treg suppress EAE by TGF-beta- and IFN-gamma-dependent mechanisms.Eur J Immunol. 2009 Dec;39(12):3423-35. doi: 10.1002/eji.200939441. Eur J Immunol. 2009. PMID: 19768696 Free PMC article.
-
Selective depletion of CD11c+ CD11b+ dendritic cells partially abrogates tolerogenic effects of intravenous MOG in murine EAE.Eur J Immunol. 2016 Oct;46(10):2454-2466. doi: 10.1002/eji.201546274. Eur J Immunol. 2016. PMID: 27338697 Free PMC article.
-
Pidotimod alleviated experimental autoimmune encephalomyelitis by regulating the balance of splenic lymphocytes.BMC Immunol. 2025 Jul 21;26(1):53. doi: 10.1186/s12865-025-00736-1. BMC Immunol. 2025. PMID: 40691766 Free PMC article.
-
Interplay between pathogenic Th17 and regulatory T cells.Ann Rheum Dis. 2007 Nov;66 Suppl 3(Suppl 3):iii87-90. doi: 10.1136/ard.2007.078527. Ann Rheum Dis. 2007. PMID: 17934104 Free PMC article. Review.
-
The dynamics of effector T cells and Foxp3+ regulatory T cells in the promotion and regulation of autoimmune encephalomyelitis.J Neuroimmunol. 2007 Nov;191(1-2):51-60. doi: 10.1016/j.jneuroim.2007.09.009. Epub 2007 Oct 3. J Neuroimmunol. 2007. PMID: 17916388 Free PMC article. Review.
Cited by
-
Mac-1 regulates disease stage-specific immunosuppression via the nitric oxide pathway in autoimmune disease.Sci Adv. 2025 May 9;11(19):eads3728. doi: 10.1126/sciadv.ads3728. Epub 2025 May 9. Sci Adv. 2025. PMID: 40344054 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- FONDECYT/ANID 1141211/Agencia Nacional de Investigación y Desarrollo
- DP2DA051912/DA/NIDA NIH HHS/United States
- DP2 DA051912/DA/NIDA NIH HHS/United States
- FONDECYT/ANID postdoc 3150133/Agencia Nacional de Investigación y Desarrollo
- National Doctoral scholarship CONICYT-CHILE 21130452/Agencia Nacional de Investigación y Desarrollo
LinkOut - more resources
Full Text Sources
Research Materials

