Soluble immune checkpoints: implications for cancer prognosis and response to immune checkpoint therapy and conventional therapies
- PMID: 38822401
- PMCID: PMC11141022
- DOI: 10.1186/s13046-024-03074-z
Soluble immune checkpoints: implications for cancer prognosis and response to immune checkpoint therapy and conventional therapies
Abstract
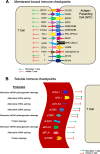
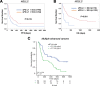
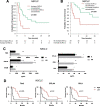
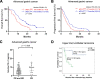
Longitudinal sampling of tumor tissue from patients with solid cancers, aside from melanoma and a few other cases, is often unfeasible, and thus may not capture the plasticity of interactions between the tumor and immune system under selective pressure of a given therapy. Peripheral blood analyses provide salient information about the human peripheral immunome while offering technical and practical advantages over traditional tumor biopsies, and should be utilized where possible alongside interrogation of the tumor. Some common blood-based biomarkers used to study the immune response include immune cell subsets, circulating tumor DNA, and protein analytes such as cytokines. With the recent explosion of immune checkpoint inhibitors (ICI) as a modality of treatment in multiple cancer types, soluble immune checkpoints have become a relevant area of investigation for peripheral immune-based biomarkers. However, the exact functions of soluble immune checkpoints and their roles in cancer for the most part remain unclear. This review discusses current literature on the production, function, and expression of nine soluble immune checkpoints - sPD-L1, sPD-1, sCTLA4, sCD80, sTIM3, sLAG3, sB7-H3, sBTLA, and sHVEM - in patients with solid tumors, and explores their role as biomarkers of response to ICI as well as to conventional therapies (chemotherapy, radiotherapy, targeted therapy, and surgery) in cancer patients.
Keywords: Biomarkers; Blood analyses; Conventional cancer therapies; Immune checkpoint inhibitors; Peripheral immunome; Soluble immune checkpoints.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Serum levels of soluble B and T lymphocyte attenuator predict overall survival in patients undergoing immune checkpoint inhibitor therapy for solid malignancies.Int J Cancer. 2021 Sep 1;149(5):1189-1198. doi: 10.1002/ijc.33610. Epub 2021 May 27. Int J Cancer. 2021. PMID: 33890289
-
Proteomic Investigation of Immune Checkpoints and Some of Their Inhibitors.Int J Mol Sci. 2024 Aug 27;25(17):9276. doi: 10.3390/ijms25179276. Int J Mol Sci. 2024. PMID: 39273224 Free PMC article. Review.
-
Immune checkpoints and cancer development: Therapeutic implications and future directions.Pathol Res Pract. 2021 Jul;223:153485. doi: 10.1016/j.prp.2021.153485. Epub 2021 May 15. Pathol Res Pract. 2021. PMID: 34022684 Review.
-
Editorial: Dynamic Biomarkers of Response to Anti-Immune Checkpoint Inhibitors in Cancer.Front Immunol. 2021 Oct 21;12:781872. doi: 10.3389/fimmu.2021.781872. eCollection 2021. Front Immunol. 2021. PMID: 34745152 Free PMC article. No abstract available.
-
Prognostic Role of Soluble and Extracellular Vesicle-Associated PD-L1, B7-H3 and B7-H4 in Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors.Cells. 2023 Mar 8;12(6):832. doi: 10.3390/cells12060832. Cells. 2023. PMID: 36980174 Free PMC article.
Cited by
-
CTLA-4-two pathways to anti-tumour immunity?Immunother Adv. 2025 Mar 7;5(1):ltaf008. doi: 10.1093/immadv/ltaf008. eCollection 2025. Immunother Adv. 2025. PMID: 40265076 Free PMC article. Review.
-
Developing a risk score using liquid biopsy biomarkers for selecting Immunotherapy responders and stratifying disease progression risk in metastatic melanoma patients.J Exp Clin Cancer Res. 2025 Feb 5;44(1):40. doi: 10.1186/s13046-025-03306-w. J Exp Clin Cancer Res. 2025. PMID: 39910579 Free PMC article.
-
A blood-based liquid biopsy analyzing soluble immune checkpoints and cytokines identifies distinct neuroendocrine tumors.J Exp Clin Cancer Res. 2025 Mar 5;44(1):82. doi: 10.1186/s13046-025-03337-3. J Exp Clin Cancer Res. 2025. PMID: 40038821 Free PMC article.
-
A dual compartment peripheral blood signature of soluble and membrane-bound immune checkpoints predicts outcome in lymphoma patients.Oncoimmunology. 2025 Dec;14(1):2543511. doi: 10.1080/2162402X.2025.2543511. Epub 2025 Aug 28. Oncoimmunology. 2025. PMID: 40873413 Free PMC article.
-
Fighting Pancreatic Cancer with a Vaccine-Based Winning Combination: Hope or Reality?Cells. 2024 Sep 16;13(18):1558. doi: 10.3390/cells13181558. Cells. 2024. PMID: 39329742 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

