A comprehensive review of in planta stable transformation strategies
- PMID: 38822403
- PMCID: PMC11140912
- DOI: 10.1186/s13007-024-01200-8
A comprehensive review of in planta stable transformation strategies
Erratum in
-
Correction: A comprehensive review of in planta stable transformation strategies.Plant Methods. 2024 Oct 15;20(1):158. doi: 10.1186/s13007-024-01282-4. Plant Methods. 2024. PMID: 39402672 Free PMC article. No abstract available.
Abstract
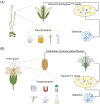
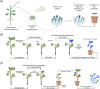
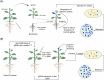
Plant transformation remains a major bottleneck to the improvement of plant science, both on fundamental and practical levels. The recalcitrant nature of most commercial and minor crops to genetic transformation slows scientific progress for a large range of crops that are essential for food security on a global scale. Over the years, novel stable transformation strategies loosely grouped under the term "in planta" have been proposed and validated in a large number of model (e.g. Arabidopsis and rice), major (e.g. wheat and soybean) and minor (e.g. chickpea and lablab bean) species. The in planta approach is revolutionary as it is considered genotype-independent, technically simple (i.e. devoid of or with minimal tissue culture steps), affordable, and easy to implement in a broad range of experimental settings. In this article, we reviewed and categorized over 300 research articles, patents, theses, and videos demonstrating the applicability of different in planta transformation strategies in 105 different genera across 139 plant species. To support this review process, we propose a classification system for the in planta techniques based on five categories and a new nomenclature for more than 30 different in planta techniques. In complement to this, we clarified some grey areas regarding the in planta conceptual framework and provided insights regarding the past, current, and future scientific impacts of these techniques. To support the diffusion of this concept across the community, this review article will serve as an introductory point for an online compendium about in planta transformation strategies that will be available to all scientists. By expanding our knowledge about in planta transformation, we can find innovative approaches to unlock the full potential of plants, support the growth of scientific knowledge, and stimulate an equitable development of plant research in all countries and institutions.
Keywords: Direct organogenesis; In planta transformation; In situ transformation; In vivo regeneration; Indirect organogenesis; Recalcitrant species.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Somssich M. A short history of plant transformation. PeerJ Prepr [Internet]. 2019;1:1–28. https://peerj.com/preprints/27556/.
-
- Padole D. Arabidopsis-a model plant. Trends Biosci. 2019;10(February):557–9.
-
- Kaur RP, Devi S. In planta transformation in plants: a review. Agric Rev. 2019;40(03):159–74.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

