The crucial role of HFM1 in regulating FUS ubiquitination and localization for oocyte meiosis prophase I progression in mice
- PMID: 38822414
- PMCID: PMC11140966
- DOI: 10.1186/s40659-024-00518-w
The crucial role of HFM1 in regulating FUS ubiquitination and localization for oocyte meiosis prophase I progression in mice
Abstract
Background: Helicase for meiosis 1 (HFM1), a putative DNA helicase expressed in germ-line cells, has been reported to be closely associated with premature ovarian insufficiency (POI). However, the underlying molecular mechanism has not been clearly elucidated. The aim of this study was to investigate the function of HFM1 in the first meiotic prophase of mouse oocytes.
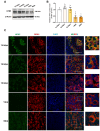
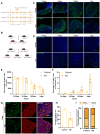
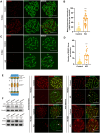
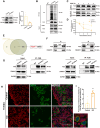
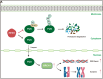
Results: The results suggested that the deficiency of HFM1 resulting in increased apoptosis and depletion of oocytes in mice, while the oocytes were arrested in the pachytene stage of the first meiotic prophase. In addition, impaired DNA double-strand break repair and disrupted synapsis were observed in the absence of HFM1. Further investigation revealed that knockout of HFM1 promoted ubiquitination and degradation of FUS protein mediated by FBXW11. Additionally, the depletion of HFM1 altered the intranuclear localization of FUS and regulated meiotic- and oocyte development-related genes in oocytes by modulating the expression of BRCA1.
Conclusions: These findings elaborated that the critical role of HFM1 in orchestrating the regulation of DNA double-strand break repair and synapsis to ensure meiosis procession and primordial follicle formation. This study provided insights into the pathogenesis of POI and highlighted the importance of HFM1 in maintaining proper meiotic function in mouse oocytes.
Keywords: FUS; HFM1; Meiosis prophase I; Oocyte; Premature ovarian failure/insufficiency.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Hfm1 participates in Golgi-associated spindle assembly and division in mouse oocyte meiosis.Cell Death Dis. 2020 Jun 30;11(6):490. doi: 10.1038/s41419-020-2697-4. Cell Death Dis. 2020. PMID: 32606310 Free PMC article.
-
FBXW24 controls female meiotic prophase progression by regulating SYCP3 ubiquitination.Clin Transl Med. 2022 Jul;12(7):e891. doi: 10.1002/ctm2.891. Clin Transl Med. 2022. PMID: 35858239 Free PMC article.
-
EZH1/2 plays critical roles in oocyte meiosis prophase I in mice.Biol Res. 2024 Nov 8;57(1):83. doi: 10.1186/s40659-024-00564-4. Biol Res. 2024. PMID: 39511641 Free PMC article.
-
The molecular control of meiotic chromosomal behavior: events in early meiotic prophase in Drosophila oocytes.Annu Rev Physiol. 2012;74:425-51. doi: 10.1146/annurev-physiol-020911-153342. Annu Rev Physiol. 2012. PMID: 22335798 Review.
-
The molecular regulatory mechanisms of meiotic arrest and resumption in Oocyte development and maturation.Reprod Biol Endocrinol. 2023 Oct 2;21(1):90. doi: 10.1186/s12958-023-01143-0. Reprod Biol Endocrinol. 2023. PMID: 37784186 Free PMC article. Review.
Cited by
-
Granulosa cells provide transcriptomic information on ovarian follicle dynamics in southern white rhinoceros.Sci Rep. 2024 Aug 20;14(1):19321. doi: 10.1038/s41598-024-70235-7. Sci Rep. 2024. PMID: 39164442 Free PMC article.
-
HFM1 is essential for the germ cell intercellular bridge transport in primordial follicle formation in mice.Cell Mol Life Sci. 2024 Dec 27;82(1):28. doi: 10.1007/s00018-024-05541-4. Cell Mol Life Sci. 2024. PMID: 39725823 Free PMC article.
References
-
- Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67(4):604–606. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

