Long-term follow-up of patients with intermediate-risk neuroblastoma treated with response- and biology-based therapy: A report from the Children's Oncology Group study ANBL0531
- PMID: 38822537
- PMCID: PMC11318088
- DOI: 10.1002/pbc.31089
Long-term follow-up of patients with intermediate-risk neuroblastoma treated with response- and biology-based therapy: A report from the Children's Oncology Group study ANBL0531
Abstract
Background: We previously reported excellent three-year overall survival (OS) for patients with newly diagnosed intermediate-risk neuroblastoma treated with a biology- and response-based algorithm on the Children's Oncology Group study ANBL0531. We now present the long-term follow-up results.
Methods: All patients who met the age, stage, and tumor biology criteria for intermediate-risk neuroblastoma were eligible. Treatment was based on prognostic biomarkers and overall response. Event-free survival (EFS) and OS were estimated by the Kaplan-Meier method.
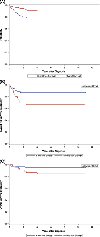
Results: The 10-year EFS and OS for the entire study cohort (n = 404) were 82.0% (95% confidence interval (CI), 77.2%-86.9%) and 94.7% (95% CI, 91.8%-97.5%), respectively. International Neuroblastoma Staging System stage 4 patients (n = 133) had inferior OS compared with non-stage 4 patients (n = 271; 10-year OS: 90.8% [95% CI, 84.5%-97.0%] vs 96.6% [95% CI, 93.9%-99.4%], p = .02). Infants with stage 4 tumors with ≥1 unfavorable biological feature (n = 47) had inferior EFS compared with those with favorable biology (n = 61; 10-year EFS: 66.8% [95% CI, 50.4%-83.3%] vs 86.9% [95% CI, 76.0%-97.8%], p = .02); OS did not differ (10-year OS: 84.4% [95% CI, 71.8%-97.0%] vs 95.0% [95% CI, 87.7%-100.0%], p = .08). Inferior EFS but not OS was observed among patients with tumors with (n = 26) versus without (n = 314) 11q loss of heterozygosity (10-year EFS: 68.4% [95% CI, 44.5%-92.2%] vs 83.9% [95% CI, 78.7%-89.2%], p = .03; 10-year OS: 88.0% [95% CI, 72.0%-100.0%] vs 95.7% [95% CI, 92.8%-98.6%], p = .09).
Conclusions: The ANBL0531 trial treatment algorithm resulted in excellent long-term survival. More effective treatments are needed for subsets of patients with unfavorable biology tumors.
Keywords: Intermediate risk; long‐term follow‐up; neuroblastoma; outcomes.
© 2024 Wiley Periodicals LLC.
Conflict of interest statement
WBL reports service on data safety monitory boards for Merck and Jubliant Draximage, and past service on a scientific advisory board for Y-mAbs Therapeutics. AN reports service on a data safety monitory board for Novartis.
AVD: stock ownership in Pfizer and Viatris; consultancy/advisory board fees from Ology Medical Education, YMabs Therapeutics, Glaxo Smith-Kline; travel/accommodation expenses from YMabs Therapeutics
Figures

References
-
- Matthay KK, Maris JM, Schleiermacher G, et al. Neuroblastoma. Primer. Nature Reviews Disease Primers. 2016;2:16078. - PubMed
-
- Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. Journal of Clinical Oncology. 1993;11(8):1466–1477. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

