Non-additive effects of electrical stimulation of the dorsolateral prefrontal cortex and the vestibular system on muscle sympathetic nerve activity in humans
- PMID: 38822824
- PMCID: PMC11208219
- DOI: 10.1007/s00221-024-06852-5
Non-additive effects of electrical stimulation of the dorsolateral prefrontal cortex and the vestibular system on muscle sympathetic nerve activity in humans
Abstract
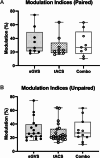
Sinusoidal galvanic vestibular stimulation (sGVS) induces robust modulation of muscle sympathetic nerve activity (MSNA) alongside perceptions of side-to-side movement, sometimes with an accompanying feeling of nausea. We recently showed that transcranial alternating current stimulation (tACS) of the dorsolateral prefrontal cortex (dlPFC) also modulates MSNA, but does not generate any perceptions. Here, we tested the hypothesis that when the two stimuli are given concurrently, the modulation of MSNA would be additive. MSNA was recorded from 11 awake participants via a tungsten microelectrode inserted percutaneously into the right common peroneal nerve at the fibular head. Sinusoidal stimuli (± 2 mA, 0.08 Hz, 100 cycles) were applied in randomised order as follows: (i) tACS of the dlPFC at electroencephalogram (EEG) site F4 and referenced to the nasion; (ii) bilateral sGVS applied to the vestibular apparatuses via the mastoid processes; and (iii) tACS and sGVS together. Previously obtained data from 12 participants supplemented the data for stimulation protocols (i) and (ii). Cross-correlation analysis revealed that each stimulation protocol caused significant modulation of MSNA (modulation index (paired data): 35.2 ± 19.4% for sGVS; 27.8 ± 15.2% for tACS), but there were no additive effects when tACS and sGVS were delivered concurrently (32.1 ± 18.5%). This implies that the vestibulosympathetic reflexes are attenuated with concurrent dlPFC stimulation. These results suggest that the dlPFC is capable of blocking the processing of vestibular inputs through the brainstem and, hence, the generation of vestibulosympathetic reflexes.
Keywords: Dorsolateral prefrontal cortex; Muscle sympathetic nerve activity; Rostral ventrolateral medulla; Transcranial electrical stimulation; Vestibular system.
© 2024. The Author(s).
Conflict of interest statement
All authors declare no conflict of interest.
Figures





Similar articles
-
Electrical stimulation of the dorsolateral prefrontal cortex inhibits vestibular signalling in humans: A BOLD fMRI study.Brain Stimul. 2025 May-Jun;18(3):627-639. doi: 10.1016/j.brs.2025.02.022. Epub 2025 Mar 5. Brain Stimul. 2025. PMID: 40054574
-
Top-down control of vestibular inputs by the dorsolateral prefrontal cortex.Exp Brain Res. 2023 Dec;241(11-12):2845-2853. doi: 10.1007/s00221-023-06722-6. Epub 2023 Oct 30. Exp Brain Res. 2023. PMID: 37902866 Free PMC article.
-
Vestibular modulation of muscle sympathetic nerve activity assessed over a 100-fold frequency range of sinusoidal galvanic vestibular stimulation.J Neurophysiol. 2019 May 1;121(5):1644-1649. doi: 10.1152/jn.00679.2018. Epub 2019 Feb 27. J Neurophysiol. 2019. PMID: 30811260
-
Vestibular Modulation of Sympathetic Nerve Activity to Muscle and Skin in Humans.Front Neurol. 2017 Jul 26;8:334. doi: 10.3389/fneur.2017.00334. eCollection 2017. Front Neurol. 2017. PMID: 28798718 Free PMC article. Review.
-
Sympathetic responses to vestibular activation in humans.Am J Physiol Regul Integr Comp Physiol. 2008 Mar;294(3):R681-8. doi: 10.1152/ajpregu.00896.2007. Epub 2008 Jan 16. Am J Physiol Regul Integr Comp Physiol. 2008. PMID: 18199586 Review.
Cited by
-
Single-pulse transcranial magnetic stimulation of the dorsolateral prefrontal cortex does not directly affect muscle sympathetic nerve activity in humans.Cereb Cortex. 2024 Dec 3;34(12):bhae484. doi: 10.1093/cercor/bhae484. Cereb Cortex. 2024. PMID: 39710610 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources

