SARA captures disparate progression and responsiveness in spinocerebellar ataxias
- PMID: 38822840
- PMCID: PMC11571887
- DOI: 10.1007/s00415-024-12475-1
SARA captures disparate progression and responsiveness in spinocerebellar ataxias
Abstract
Background: The Scale for Assessment and Rating of Ataxia (SARA) is a widely used clinical scale to assess cerebellar ataxia but faces some criticisms about the relevancy of all its items.
Objectives: To prepare for future clinical trials, we analyzed the progression of SARA and its items in several polyQ spinocerebellar ataxias (SCA) from various cohorts.
Methods: We included data from patients with SCA1, SCA2, SCA3, and SCA6 from four cohorts (EUROSCA, RISCA, CRC-SCA, and SPATAX) for a total of 850 carriers and 3431 observations. Longitudinal progression of the SARA and its items was measured. Cohort, stage and genetic effects were tested. We looked at the respective contribution of each item to the total scale. Sensitivity to change of the scale and the impact of item removal was evaluated by calculating sample sizes needed in various scenarios.
Results: Longitudinal progression was significantly different between cohorts in SCA1, SCA2 and SCA3, the EUROSCA cohort having the fastest progression. Advanced-stage patients were progressing slower in SCA2 and SCA6. Items were not contributing equally to the full scale through ataxia severity: gait, stance, hand movement, and heel-shin contributed the most in the early stage, and finger-chase, nose-finger, and sitting in later stages. Few items drove the sensitivity to the change of SARA, but changes in the scale structure could not improve its sensitivity in all populations.
Conclusion: SARA and its item's progression pace showed high heterogeneity across cohorts and SCAs. However, no combinations of items improved the responsiveness in all SCAs or populations taken separately.
Keywords: Clinical score; Natural history; Spinocerebellar ataxia.
© 2024. Springer-Verlag GmbH Germany, part of Springer Nature.
Figures
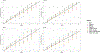
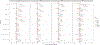

References
-
- Ashizawa T, Figueroa KP, Perlman SL, Gomez CM, Wilmot GR, Schmahmann JD, Ying SH, Zesiewicz TA, Paulson HL, Shakkottai VG, Bushara KO, Kuo S-H, Geschwind MD, Xia G, Mazzoni P, Krischer JP, Cuthbertson D, Holbert A, Ferguson JH, Pulst SM, Subramony S, 2013. Clinical characteristics of patients with spinocerebellar ataxias 1, 2, 3 and 6 in the US; a prospective observational study. Orphanet J. Rare Dis. 8, 177. 10.1186/1750-1172-8-177 - DOI - PMC - PubMed
-
- Coarelli G, Heinzmann A, Ewenczyk C, Fischer C, Chupin M, Monin M-L, Hurmic H, Calvas F, Calvas P, Goizet C, Thobois S, Anheim M, Nguyen K, Devos D, Verny C, Ricigliano VAG, Mangin J-F, Brice A, Tezenas du Montcel S, Durr A, 2022. Safety and efficacy of riluzole in spinocerebellar ataxia type 2 in France (ATRIL): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 21, 225–233. 10.1016/S1474-4422(21)00457-9 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

