Noise or signal? Spontaneous activity of dorsal horn neurons: patterns and function in health and disease
- PMID: 38822875
- PMCID: PMC11271371
- DOI: 10.1007/s00424-024-02971-8
Noise or signal? Spontaneous activity of dorsal horn neurons: patterns and function in health and disease
Abstract
Spontaneous activity refers to the firing of action potentials by neurons in the absence of external stimulation. Initially considered an artifact or "noise" in the nervous system, it is now recognized as a potential feature of neural function. Spontaneous activity has been observed in various brain areas, in experimental preparations from different animal species, and in live animals and humans using non-invasive imaging techniques. In this review, we specifically focus on the spontaneous activity of dorsal horn neurons of the spinal cord. We use a historical perspective to set the basis for a novel classification of the different patterns of spontaneous activity exhibited by dorsal horn neurons. Then we examine the origins of this activity and propose a model circuit to explain how the activity is generated and transmitted to the dorsal horn. Finally, we discuss possible roles of this activity during development and during signal processing under physiological conditions and pain states. By analyzing recent studies on the spontaneous activity of dorsal horn neurons, we aim to shed light on its significance in sensory processing. Understanding the different patterns of activity, the origins of this activity, and the potential roles it may play, will contribute to our knowledge of sensory mechanisms, including pain, to facilitate the modeling of spinal circuits and hopefully to explore novel strategies for pain treatment.
Keywords: Firing pattern; Sensory processing; Spike trains; Spinal cord.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
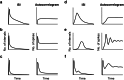
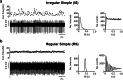

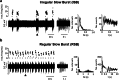
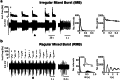

Similar articles
-
Alterations in evoked and spontaneous activity of dorsal horn wide dynamic range neurons in pathological pain: a systematic review and analysis.Pain. 2019 Oct;160(10):2199-2209. doi: 10.1097/j.pain.0000000000001632. Pain. 2019. PMID: 31149976
-
Inhibition Mediated by Glycinergic and GABAergic Receptors on Excitatory Neurons in Mouse Superficial Dorsal Horn Is Location-Specific but Modified by Inflammation.J Neurosci. 2017 Mar 1;37(9):2336-2348. doi: 10.1523/JNEUROSCI.2354-16.2017. Epub 2017 Jan 27. J Neurosci. 2017. PMID: 28130358 Free PMC article.
-
Multiscale Computer Model of the Spinal Dorsal Horn Reveals Changes in Network Processing Associated with Chronic Pain.J Neurosci. 2022 Apr 13;42(15):3133-3149. doi: 10.1523/JNEUROSCI.1199-21.2022. Epub 2022 Mar 1. J Neurosci. 2022. PMID: 35232767 Free PMC article.
-
Network model of nociceptive processing in the superficial spinal dorsal horn reveals mechanisms of hyperalgesia, allodynia, and spinal cord stimulation.J Neurophysiol. 2023 Nov 1;130(5):1103-1117. doi: 10.1152/jn.00186.2023. Epub 2023 Sep 20. J Neurophysiol. 2023. PMID: 37727912
-
Neuronal hyperexcitability: a substrate for central neuropathic pain after spinal cord injury.Curr Pain Headache Rep. 2011 Jun;15(3):215-22. doi: 10.1007/s11916-011-0186-2. Curr Pain Headache Rep. 2011. PMID: 21387163 Review.
Cited by
-
Central terminals of primary afferents coordinate the spontaneous activity of dorsal horn neurons.J Physiol. 2025 Jun;603(12):3589-3603. doi: 10.1113/JP287970. Epub 2025 Jun 7. J Physiol. 2025. PMID: 40483563 Free PMC article.
-
[Research on the relationship between resting-state spontaneous electroencephalography and task-evoked electroencephalography].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2025 Jun 25;42(3):620-627. doi: 10.7507/1001-5515.202501021. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2025. PMID: 40566786 Free PMC article. Review. Chinese.
-
An electrophysiologist's guide to dorsal horn excitability and pain.Front Cell Neurosci. 2025 Apr 2;19:1548252. doi: 10.3389/fncel.2025.1548252. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40241846 Free PMC article. Review.
References
-
- Abraira VE, Kuehn ED, Chirila AM, Springel MW, Toliver AA, Zimmerman AL, Orefice LL, Boyle KA, Bai L, Song BJ, Bashista KA, O’Neill TG, Zhuo J, Tsan C, Hoynoski J, Rutlin M, Kus L, Niederkofler V, Watanabe M, Dymecki SM, Nelson SB, Heintz N, Hughes DI, Ginty DD (2017) The cellular and synaptic architecture of the mechanosensory dorsal horn. Cell 168:295-310.e19. 10.1016/j.cell.2016.12.010 10.1016/j.cell.2016.12.010 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

