Unlocking Arene Phosphorescence in Bismuth-Organic Materials
- PMID: 38823026
- PMCID: PMC11186004
- DOI: 10.1021/acs.inorgchem.4c00606
Unlocking Arene Phosphorescence in Bismuth-Organic Materials
Abstract
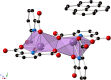
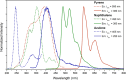
Three novel bismuth-organic compounds, with the general formula [Bi2(HPDC)2(PDC)2]·(arene)·2H2O (H2PDC = 2,6-pyridinedicarboxylic acid; arene = pyrene, naphthalene, and azulene), that consist of neutral dinuclear Bi-pyridinedicarboxylate complexes and outer coordination sphere arene molecules were synthesized and structurally characterized. The structures of all three phases exhibit strong π-π stacking interactions between the Bi-bound PDC/HPDC and outer sphere organic molecules; these interactions effectively sandwich the arene molecules between bismuth complexes and thereby prevent molecular vibrations. Upon UV irradiation, the compounds containing pyrene and naphthalene displayed red and green emission, respectively, with quantum yields of 1.3(2) and 30.8(4)%. The emission was found to originate from the T1 → S0 transition of the corresponding arene and result in phosphorescence characteristic of the arene employed. By comparison, the azulene-containing compound displayed very weak blue-purple phosphorescence of unknown origin and is a rare example of T2 → S0 emission from azulene. The pyrene- and naphthalene-containing compounds both display radioluminescence, with intensities of 11 and 38% relative to bismuth germanate, respectively. Collectively, these results provide further insights into the structure-property relationships that underpin luminescence from Bi-based materials and highlight the utility of Bi-organic molecules in the realization of organic emission.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Kumar P.; Singh S.; Gupta I.; Kumar V.; Singh D. Preparation and luminescence behaviour of perovskite LaAlO3:Tb3+ nanophosphors for innovative displays. Optik 2022, 267, 169709.10.1016/j.ijleo.2022.169709. - DOI
-
- Liu Y.; Li C.; Ren Z.; Yan S.; Bryce M. R. All-organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Nat. Rev. Mater. 2018, 3 (4), 18020.10.1038/natrevmats.2018.20. - DOI
-
- Shi Y.; Wang Z.; Meng T.; Yuan T.; Ni R.; Li Y.; Li X.; Zhang Y.; Tan Z.; Lei S.; Fan L. Red phosphorescent carbon quantum dot organic framework-based electroluminescent light-emitting diodes exceeding 5% external quantum efficiency. J. Am. Chem. Soc. 2021, 143 (45), 18941–18951. 10.1021/jacs.1c07054. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

