Targeting F-actin stress fibers to suppress the dedifferentiated phenotype in chondrocytes
- PMID: 38823166
- PMCID: PMC11610718
- DOI: 10.1016/j.ejcb.2024.151424
Targeting F-actin stress fibers to suppress the dedifferentiated phenotype in chondrocytes
Abstract
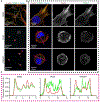
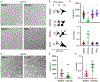
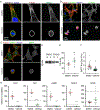
Actin is a central mediator of the chondrocyte phenotype. Monolayer expansion of articular chondrocytes on tissue culture polystyrene, for cell-based repair therapies, leads to chondrocyte dedifferentiation. During dedifferentiation, chondrocytes spread and filamentous (F-)actin reorganizes from a cortical to a stress fiber arrangement causing a reduction in cartilage matrix expression and an increase in fibroblastic matrix and contractile molecule expression. While the downstream mechanisms regulating chondrocyte molecular expression by alterations in F-actin organization have become elucidated, the critical upstream regulators of F-actin networks in chondrocytes are not completely known. Tropomyosin (TPM) and the RhoGTPases are known regulators of F-actin networks. The main purpose of this study is to elucidate the regulation of passaged chondrocyte F-actin stress fiber networks and cell phenotype by the specific TPM, TPM3.1, and the RhoGTPase, CDC42. Our results demonstrated that TPM3.1 associates with cortical F-actin and stress fiber F-actin in primary and passaged chondrocytes, respectively. In passaged cells, we found that pharmacological TPM3.1 inhibition or siRNA knockdown causes F-actin reorganization from stress fibers back to cortical F-actin and causes an increase in G/F-actin. CDC42 inhibition also causes formation of cortical F-actin. However, pharmacological CDC42 inhibition, but not TPM3.1 inhibition, leads to the re-association of TPM3.1 with cortical F-actin. Both TPM3.1 and CDC42 inhibition, as well as TPM3.1 knockdown, reduces nuclear localization of myocardin related transcription factor, which suppresses dedifferentiated molecule expression. We confirmed that TPM3.1 or CDC42 inhibition partially redifferentiates passaged cells by reducing fibroblast matrix and contractile expression, and increasing chondrogenic SOX9 expression. A further understanding on the regulation of F-actin in passaged cells may lead into new insights to stimulate cartilage matrix expression in cells for regenerative therapies.
Keywords: Actin; CDC42; Chondrocytes; Dedifferentiation; MRTF; Stress fibers; TPM3.1; cortical actin.
Copyright © 2024 The Authors. Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None
Figures

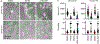





Update of
-
Targeting F-actin stress fibers to suppress the dedifferentiated phenotype in chondrocytes.bioRxiv [Preprint]. 2023 Dec 9:2023.12.08.570865. doi: 10.1101/2023.12.08.570865. bioRxiv. 2023. Update in: Eur J Cell Biol. 2024 Jun;103(2):151424. doi: 10.1016/j.ejcb.2024.151424. PMID: 38106134 Free PMC article. Updated. Preprint.
Similar articles
-
Targeting the reorganization of F-actin for cell-based implantation cartilage repair therapies.Differentiation. 2025 May-Jun;143:100847. doi: 10.1016/j.diff.2025.100847. Epub 2025 Mar 1. Differentiation. 2025. PMID: 40068531 Review.
-
Targeting F-actin stress fibers to suppress the dedifferentiated phenotype in chondrocytes.bioRxiv [Preprint]. 2023 Dec 9:2023.12.08.570865. doi: 10.1101/2023.12.08.570865. bioRxiv. 2023. Update in: Eur J Cell Biol. 2024 Jun;103(2):151424. doi: 10.1016/j.ejcb.2024.151424. PMID: 38106134 Free PMC article. Updated. Preprint.
-
MRTF-A signaling regulates the acquisition of the contractile phenotype in dedifferentiated chondrocytes.Matrix Biol. 2017 Oct;62:3-14. doi: 10.1016/j.matbio.2016.10.004. Epub 2016 Oct 14. Matrix Biol. 2017. PMID: 27751947
-
Articular Chondrocyte Phenotype Regulation through the Cytoskeleton and the Signaling Processes That Originate from or Converge on the Cytoskeleton: Towards a Novel Understanding of the Intersection between Actin Dynamics and Chondrogenic Function.Int J Mol Sci. 2021 Mar 23;22(6):3279. doi: 10.3390/ijms22063279. Int J Mol Sci. 2021. PMID: 33807043 Free PMC article. Review.
-
Expression of type I collagen and tenascin C is regulated by actin polymerization through MRTF in dedifferentiated chondrocytes.FEBS Lett. 2014 Oct 16;588(20):3677-84. doi: 10.1016/j.febslet.2014.08.012. Epub 2014 Aug 19. FEBS Lett. 2014. PMID: 25150168
Cited by
-
Actin Polymerization Status Regulates Tendon Homeostasis through Myocardin-Related Transcription Factor-A.bioRxiv [Preprint]. 2024 Aug 26:2024.08.26.609684. doi: 10.1101/2024.08.26.609684. bioRxiv. 2024. Update in: Cytoskeleton (Hoboken). 2024 Nov 27. doi: 10.1002/cm.21962. PMID: 39253450 Free PMC article. Updated. Preprint.
-
TIMP-1 enhances Akt and BDNF signaling in neurons to reduce synaptic and cognitive deficits in 5xFAD mouse model of Alzheimer's disease.Acta Neuropathol Commun. 2025 Aug 19;13(1):178. doi: 10.1186/s40478-025-02060-6. Acta Neuropathol Commun. 2025. PMID: 40830532 Free PMC article.
-
The Effects of Hypothermic Storage on Passaged Chondrocyte Viability and Redifferentiation Potential.bioRxiv [Preprint]. 2025 Jun 26:2025.06.20.660742. doi: 10.1101/2025.06.20.660742. bioRxiv. 2025. PMID: 40666901 Free PMC article. Preprint.
-
Targeting the reorganization of F-actin for cell-based implantation cartilage repair therapies.Differentiation. 2025 May-Jun;143:100847. doi: 10.1016/j.diff.2025.100847. Epub 2025 Mar 1. Differentiation. 2025. PMID: 40068531 Review.
-
TNF-Alpha Inhibitor Prevents Cigarette Smoke Extract-Induced Cell Death in Osteoarthritis-Derived Chondrocytes in Culture.Cells. 2025 Mar 25;14(7):489. doi: 10.3390/cells14070489. Cells. 2025. PMID: 40214444 Free PMC article.
References
-
- Asparuhova MB, Ferralli J, Chiquet M, Chiquet-Ehrismann R, 2011. The transcriptional regulator megakaryoblastic leukemia-1 mediates serum response factor-independent activation of tenascin-C transcription by mechanical stress. FASEB J 25, 3477–3488. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

