First analytical confirmation of drug-induced crystal nephropathy in felines caused by GS-441524, the active metabolite of Remdesivir
- PMID: 38823223
- PMCID: PMC11229044
- DOI: 10.1016/j.jpba.2024.116248
First analytical confirmation of drug-induced crystal nephropathy in felines caused by GS-441524, the active metabolite of Remdesivir
Abstract
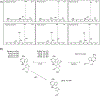
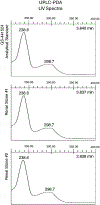
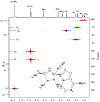
GS-441524 is an adenosine nucleoside antiviral demonstrating significant efficacy in the treatment of feline infectious peritonitis (FIP), an otherwise fatal illness, resulting from infection with feline coronavirus. However, following the emergence of COVID-19, veterinary development was halted, and Gilead pursued clinical development of a GS-441524 pro-drug, resulting in the approval of Remdesivir under an FDA emergency use authorization. Despite lack of regulatory approval, GS-441524 is available without a prescription through various unlicensed online distributors and is commonly purchased by pet owners for the treatment of FIP. Herein, we report data obtained from the analytical characterization of two feline renal calculi, demonstrating the propensity for GS-441524 to cause renal toxicity through drug-induced crystal nephropathy in vivo. As definitive diagnosis of drug-induced crystal nephropathy requires confirmation of the lithogenic material to accurately attribute a mechanism of toxicity, renal stone composition and crystalline matrix were characterized using ultra-performance liquid chromatography photodiode array detection (UPLC-PDA), ultra-performance liquid chromatography mass spectrometry (LCMS), nuclear magnetic resonance (NMR) spectroscopy, X-ray powder diffraction (XRD), and Fourier-transform infrared spectroscopy (FTIR). This work serves to provide the first analytical confirmation of GS-441524-induced crystal nephropathy in an effort to support toxicologic identification of adverse renal effects caused by administration of GS-441524 or any pro-drug thereof.
Keywords: Coronavirus; Crystal nephropathy; Feline infectious peritonitis; GS-441524; Remdesivir; Renal toxicity.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Yarlagadda SG, Perazella MA, Drug-induced crystal nephropathy: an update, Expert Opin Drug Saf, 7 (2008) 147–158. - PubMed
-
- Perazella MA, Drug-induced nephropathy: an update, Expert Opin Drug Saf, 4 (2005) 689–706. - PubMed
-
- Daudon M, Frochot V, Bazin D, Jungers P, Drug-Induced Kidney Stones and Crystalline Nephropathy: Pathophysiology, Prevention and Treatment, Drugs, 78 (2018) 163–201. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

