DLK1-DIO3 region as a source of tumor suppressor miRNAs in papillary thyroid carcinoma
- PMID: 38823258
- PMCID: PMC11176784
- DOI: 10.1016/j.tranon.2023.101849
DLK1-DIO3 region as a source of tumor suppressor miRNAs in papillary thyroid carcinoma
Abstract
Background: In previous studies, we demonstrated the downregulation of several miRNAs from the DLK1-DIO3 genomic region in papillary thyroid carcinoma (PTC). Due to the large number of miRNAs within this region, the individual contribution of these molecules to PTC development and progression remains unclear.
Objective: In this study, we aimed to clarify the contribution of DLK1-DIO3-derived miRNAs to PTC.
Methods: We used different computational approaches and in vitro resources to assess the biological processes and signaling pathways potentially modulated by these miRNAs.
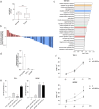
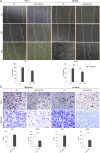
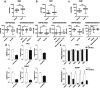
Results: Our analysis suggests that, out of more than 100 mature miRNAs originated from the DLK1-DIO3 region, a set of 12 miRNAs accounts for most of the impact on PTC development and progression, cooperating to modulate distinct cancer-relevant biological processes, such as cell migration, extracellular matrix remodeling, and signal transduction. The restoration of the expression of one of these miRNAs (miR-485-5p) in a BRAFT199A-positive PTC cell line impaired proliferation and migration, suppressing the expression of GAB2 and RAC1, validated miR-485-5p targets.
Conclusions: Overall, our results shed light on the role of the DLK1-DIO3 region, which harbors promising tumor suppressor miRNAs in thyroid cancer, and open prospects for the functional exploration of these miRNAs as therapeutic targets for PTC.
Keywords: DLK1-DIO3 region; MicroRNA; Papillary thyroid cancer; miR-485–5p.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest All the following authors declare that there is no financial/personal interest or belief that could affect their objectivity
Figures


Similar articles
-
MiR-495-3p regulates cell migration and invasion in papillary thyroid carcinoma.Front Oncol. 2023 Jan 26;13:1039654. doi: 10.3389/fonc.2023.1039654. eCollection 2023. Front Oncol. 2023. PMID: 36776296 Free PMC article.
-
Down-regulation of 14q32-encoded miRNAs and tumor suppressor role for miR-654-3p in papillary thyroid cancer.Oncotarget. 2017 Feb 7;8(6):9597-9607. doi: 10.18632/oncotarget.14162. Oncotarget. 2017. PMID: 28030816 Free PMC article.
-
Male-specific coordinated changes in expression of miRNA genes, but not other genes within the DLK1-DIO3 locus in multiple sclerosis.Gene. 2022 Aug 20;836:146676. doi: 10.1016/j.gene.2022.146676. Epub 2022 Jun 14. Gene. 2022. PMID: 35714798
-
miR-451a is underexpressed and targets AKT/mTOR pathway in papillary thyroid carcinoma.Oncotarget. 2016 Mar 15;7(11):12731-47. doi: 10.18632/oncotarget.7262. Oncotarget. 2016. PMID: 26871295 Free PMC article. Review.
-
A Hearty Dose of Noncoding RNAs: The Imprinted DLK1-DIO3 Locus in Cardiac Development and Disease.J Cardiovasc Dev Dis. 2018 Jul 10;5(3):37. doi: 10.3390/jcdd5030037. J Cardiovasc Dev Dis. 2018. PMID: 29996488 Free PMC article. Review.
Cited by
-
MiR-495-3p regulates cell migration and invasion in papillary thyroid carcinoma.Front Oncol. 2023 Jan 26;13:1039654. doi: 10.3389/fonc.2023.1039654. eCollection 2023. Front Oncol. 2023. PMID: 36776296 Free PMC article.
-
Epigenetic Regulation of DLK1-DIO3 Region in Thyroid Carcinoma.Cells. 2024 Jun 8;13(12):1001. doi: 10.3390/cells13121001. Cells. 2024. PMID: 38920632 Free PMC article.
References
-
- Suarez H.G., Du Villard J.A., Caillou B., et al. Detection of activated ras oncogenes in human thyroid carcinomas. Oncogene. 1988;2(4) - PubMed
-
- Nikiforova M.N., Kimura E.T., Gandhi M., et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J. Clin. Endocrinol. Metab. 2003;88(11):5399–5404. doi: 10.1210/jc.2003-030838. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

