De novo and salvage purine synthesis pathways across tissues and tumors
- PMID: 38823389
- PMCID: PMC11246224
- DOI: 10.1016/j.cell.2024.05.011
De novo and salvage purine synthesis pathways across tissues and tumors
Abstract
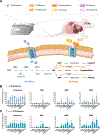
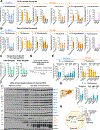
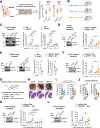
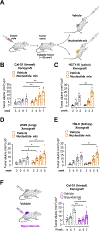
Purine nucleotides are vital for RNA and DNA synthesis, signaling, metabolism, and energy homeostasis. To synthesize purines, cells use two principal routes: the de novo and salvage pathways. Traditionally, it is believed that proliferating cells predominantly rely on de novo synthesis, whereas differentiated tissues favor the salvage pathway. Unexpectedly, we find that adenine and inosine are the most effective circulating precursors for supplying purine nucleotides to tissues and tumors, while hypoxanthine is rapidly catabolized and poorly salvaged in vivo. Quantitative metabolic analysis demonstrates comparative contribution from de novo synthesis and salvage pathways in maintaining purine nucleotide pools in tumors. Notably, feeding mice nucleotides accelerates tumor growth, while inhibiting purine salvage slows down tumor progression, revealing a crucial role of the salvage pathway in tumor metabolism. These findings provide fundamental insights into how normal tissues and tumors maintain purine nucleotides and highlight the significance of purine salvage in cancer.
Keywords: cancer metabolism; de novo purine synthesis; in vivo isotope tracing; nucleotide diet; nucleotide metabolism; purine bases; purine degradation; purine salvage; tissue; tumor growth.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests R.J.D. is an advisor for Agios Pharmaceuticals and Vida Ventures. S.J.M. is an advisor for Frequency Therapeutics and Protein Fluidics, as well as a stockholder in G1 Therapeutics and Mereo Biopharma. H.Z. has a sponsored research agreement with Alnylam Pharmaceuticals, consults for Flagship Pioneering and Chroma Medicines, and serves on the SAB of Ubiquitix. J.B. is an employee/paid consultant for Arrowhead, Calithera, Esai, Exelixis, and Johnson & Johnson and reports receiving commercial research grants from Arrowhead.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

