Inflammatory risk and cardiovascular events in patients without obstructive coronary artery disease: the ORFAN multicentre, longitudinal cohort study
- PMID: 38823406
- PMCID: PMC11664027
- DOI: 10.1016/S0140-6736(24)00596-8
Inflammatory risk and cardiovascular events in patients without obstructive coronary artery disease: the ORFAN multicentre, longitudinal cohort study
Abstract
Background: Coronary computed tomography angiography (CCTA) is the first line investigation for chest pain, and it is used to guide revascularisation. However, the widespread adoption of CCTA has revealed a large group of individuals without obstructive coronary artery disease (CAD), with unclear prognosis and management. Measurement of coronary inflammation from CCTA using the perivascular fat attenuation index (FAI) Score could enable cardiovascular risk prediction and guide the management of individuals without obstructive CAD. The Oxford Risk Factors And Non-invasive imaging (ORFAN) study aimed to evaluate the risk profile and event rates among patients undergoing CCTA as part of routine clinical care in the UK National Health Service (NHS); to test the hypothesis that coronary arterial inflammation drives cardiac mortality or major adverse cardiac events (MACE) in patients with or without CAD; and to externally validate the performance of the previously trained artificial intelligence (AI)-Risk prognostic algorithm and the related AI-Risk classification system in a UK population.
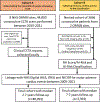
Methods: This multicentre, longitudinal cohort study included 40 091 consecutive patients undergoing clinically indicated CCTA in eight UK hospitals, who were followed up for MACE (ie, myocardial infarction, new onset heart failure, or cardiac death) for a median of 2·7 years (IQR 1·4-5·3). The prognostic value of FAI Score in the presence and absence of obstructive CAD was evaluated in 3393 consecutive patients from the two hospitals with the longest follow-up (7·7 years [6·4-9·1]). An AI-enhanced cardiac risk prediction algorithm, which integrates FAI Score, coronary plaque metrics, and clinical risk factors, was then evaluated in this population.
Findings: In the 2·7 year median follow-up period, patients without obstructive CAD (32 533 [81·1%] of 40 091) accounted for 2857 (66·3%) of the 4307 total MACE and 1118 (63·7%) of the 1754 total cardiac deaths in the whole of Cohort A. Increased FAI Score in all the three coronary arteries had an additive impact on the risk for cardiac mortality (hazard ratio [HR] 29·8 [95% CI 13·9-63·9], p<0·001) or MACE (12·6 [8·5-18·6], p<0·001) comparing three vessels with an FAI Score in the top versus bottom quartile for each artery. FAI Score in any coronary artery predicted cardiac mortality and MACE independently from cardiovascular risk factors and the presence or extent of CAD. The AI-Risk classification was positively associated with cardiac mortality (6·75 [5·17-8·82], p<0·001, for very high risk vs low or medium risk) and MACE (4·68 [3·93-5·57], p<0·001 for very high risk vs low or medium risk). Finally, the AI-Risk model was well calibrated against true events.
Interpretation: The FAI Score captures inflammatory risk beyond the current clinical risk stratification and CCTA interpretation, particularly among patients without obstructive CAD. The AI-Risk integrates this information in a prognostic algorithm, which could be used as an alternative to traditional risk factor-based risk calculators.
Funding: British Heart Foundation, NHS-AI award, Innovate UK, National Institute for Health and Care Research, and the Oxford Biomedical Research Centre.
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests AT has received research funding from National Institute of Health and Care Research (NIHR) Oxford Health Biomedical Research Center and NIHR Applied Research Collaboration Oxford. AKa has received grants from Lantheus Medical USA and honoraria from Bracco UK/Philips Medical. BM has received honoraria from Chiesi, Sanofi, Novartis, and Boston Scientific. CA has a leadership role in British Atherosclerosis Society, and participates in several European Commission Marie Curie panels, has received honoraria from Amarin and Covance, and has received consulting fees from Slience Therapeutics. DA has a leadership role in the Spontaneous Coronary Artery Dissection Study group, is inventor of patents related to a cardiac assist device (EP3277337A1, PCT/GB2017/050877), and has received grant support from AstraZeneca and Abbott Vascular, and consulting fees from General Electric. EM has received research support from the NHS AI award. EN has a leadership role in the Society of Cardiovascular Computed Tomography and has received consulting fees from Caristo Diagnostics. EKO is a stock option holder of Caristo Diagnostics, is co-founder of Evidence2Health, and is inventor of patents (WO2018078395A1, WO2020058713A1, US17/720,068, 63/619,241, 63/177,117, 63/580,137, 63/606,203, and 63/562,335). JD has a leadership role and has received consulting fees from Novo Nordisk, has received honoraria from Amgen, Boehringer Ingelheim, Merck, Pfizer, Aegerion, Novartis, Sanofi, Takeda, Novo Nordisk, and Bayer. JR has a leadership role in Heart & Lung Imaging, has received consulting fees from NHSX and HeartFlow, and honoraria from Sanofi, Aidence, and 4-C. KMC has received consulting fees from Caristo Diagnostics. MD has received consulting fees from Bristol Myers Squibb, Tenaya Therapeutics, and VizAL, and has participated on an advisory board for Caristo Diagnostics. NSa receives royalties from a patent (PCT/GB2015/052359). PL has received research support from National Heart, Lung and Blood Institute, Simard Fund, and RRM Charitable Fund, grants from Novartis, Novo Nordisk, and Genentech, honoraria from Pri-Med and Medtelligence, has a leadership role in XBiotech, is the inventor of patents (US20240043525A1, US20220041710A1 and US20220389090A1), and has advisory roles for Novartis, DalCor, XBiotech, TenSixteen Bio, and Soley Therapeutics. RB has a leadership role in the Society of Cardiovascular Computed Tomography, has received grants from Amgen, Novartis, and Nanox AI, and consulting fees from Caristo Diagnostics and Heartflow. SEP has a leadership role for the European Association of Cardiovascular Imaging, has received consulting fees from Circle Cardiovascular Imaging, and holds an advisory role for PROTEUS Trial. PT, YS, and MS are employees of Caristo Diagnostics. SN, KMC, and CA are founders, shareholders, and directors of Caristo Diagnostics, a CT-image analysis company. CA is the inventor of patents US10695023B2, US11393137B2, GB2018/1818049.7, GR20180100490, and GR20180100510. ASA, SN, and KMC are co-inventors of patent US10695023B2. These are licensed to Caristo Diagnostics. All other authors declare no competing interests.
Figures




Comment in
-
Beyond the AJR: Pericoronary Fat Attenuation Index Measurements With CT-The Need for Standardization.AJR Am J Roentgenol. 2025 May;224(5):e2431946. doi: 10.2214/AJR.24.31946. Epub 2024 Aug 28. AJR Am J Roentgenol. 2025. PMID: 39194312 No abstract available.
References
-
- The National Institute for Health and Care Excellence (NICE). CVD risk assessment and management (CG181). National Institute for Health and Care Excellence; 2020.
-
- Corrigendum to: 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Eur Heart J 2022; 43(42): 4468. - PubMed
-
- The National Institute for Health and Care Excellence (NICE). NICE Guidance for Stable Chest Pain Patients (CG95 & MTG32) to Appropriately Diagnose Patients with Suspected Coronary Artery Disease. 2019.
-
- SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015; 385(9985): 2383–91. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 HL163099/HL/NHLBI NIH HHS/United States
- R01 HL134892/HL/NHLBI NIH HHS/United States
- R01 HL034636/HL/NHLBI NIH HHS/United States
- R01 AG063839/AG/NIA NIH HHS/United States
- CH/F/21/90009/BHF_/British Heart Foundation/United Kingdom
- TG/19/2/34831/BHF_/British Heart Foundation/United Kingdom
- CH/16/1/32013/BHF_/British Heart Foundation/United Kingdom
- R01 HL151627/HL/NHLBI NIH HHS/United States
- R01 HL166538/HL/NHLBI NIH HHS/United States
- R01 HL157073/HL/NHLBI NIH HHS/United States
- CH/09/002/26360/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

