Area postrema neurons mediate interleukin-6 function in cancer cachexia
- PMID: 38824130
- PMCID: PMC11144211
- DOI: 10.1038/s41467-024-48971-1
Area postrema neurons mediate interleukin-6 function in cancer cachexia
Abstract
Interleukin-6 (IL-6) has been long considered a key player in cancer cachexia. It is believed that sustained elevation of IL-6 production during cancer progression causes brain dysfunctions, which ultimately result in cachexia. However, how peripheral IL-6 influences the brain remains poorly understood. Here we show that neurons in the area postrema (AP), a circumventricular structure in the hindbrain, is a critical mediator of IL-6 function in cancer cachexia in male mice. We find that circulating IL-6 can rapidly enter the AP and activate neurons in the AP and its associated network. Peripheral tumor, known to increase circulating IL-6, leads to elevated IL-6 in the AP, and causes potentiated excitatory synaptic transmission onto AP neurons and AP network hyperactivity. Remarkably, neutralization of IL-6 in the brain of tumor-bearing mice with an anti-IL-6 antibody attenuates cachexia and the hyperactivity in the AP network, and markedly prolongs lifespan. Furthermore, suppression of Il6ra, the gene encoding IL-6 receptor, specifically in AP neurons with CRISPR/dCas9 interference achieves similar effects. Silencing Gfral-expressing AP neurons also attenuates cancer cachectic phenotypes and AP network hyperactivity. Our study identifies a central mechanism underlying the function of peripheral IL-6, which may serve as a target for treating cancer cachexia.
© 2024. The Author(s).
Conflict of interest statement
Q.S., D.V.D.L. and B.L. are listed as inventors on a patent application concerning the development of new strategies for treating cancer cachexia. All other authors declare no competing interests.
Figures
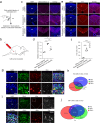
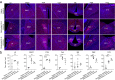
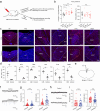
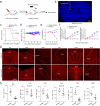
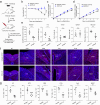
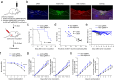
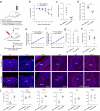
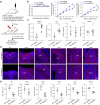
Update of
-
Area postrema neurons mediate interleukin-6 function in cancer-associated cachexia.bioRxiv [Preprint]. 2023 Jan 14:2023.01.12.523716. doi: 10.1101/2023.01.12.523716. bioRxiv. 2023. Update in: Nat Commun. 2024 Jun 1;15(1):4682. doi: 10.1038/s41467-024-48971-1. PMID: 36711916 Free PMC article. Updated. Preprint.
Similar articles
-
Area postrema neurons mediate interleukin-6 function in cancer-associated cachexia.bioRxiv [Preprint]. 2023 Jan 14:2023.01.12.523716. doi: 10.1101/2023.01.12.523716. bioRxiv. 2023. Update in: Nat Commun. 2024 Jun 1;15(1):4682. doi: 10.1038/s41467-024-48971-1. PMID: 36711916 Free PMC article. Updated. Preprint.
-
Sex differences in the relationship of IL-6 signaling to cancer cachexia progression.Biochim Biophys Acta. 2015 May;1852(5):816-25. doi: 10.1016/j.bbadis.2014.12.015. Epub 2014 Dec 30. Biochim Biophys Acta. 2015. PMID: 25555992 Free PMC article.
-
Increased thrombin generation in a mouse model of cancer cachexia is partially interleukin-6 dependent.J Thromb Haemost. 2017 Mar;15(3):477-486. doi: 10.1111/jth.13612. Epub 2017 Feb 14. J Thromb Haemost. 2017. PMID: 28058802
-
Role of interleukin-6 in cachexia: therapeutic implications.Curr Opin Support Palliat Care. 2014 Dec;8(4):321-7. doi: 10.1097/SPC.0000000000000091. Curr Opin Support Palliat Care. 2014. PMID: 25319274 Free PMC article. Review.
-
Exercise as an anti-inflammatory therapy for cancer cachexia: a focus on interleukin-6 regulation.Am J Physiol Regul Integr Comp Physiol. 2020 Feb 1;318(2):R296-R310. doi: 10.1152/ajpregu.00147.2019. Epub 2019 Dec 11. Am J Physiol Regul Integr Comp Physiol. 2020. PMID: 31823669 Review.
Cited by
-
Neural pathways of nausea and roles in energy balance.Curr Opin Neurobiol. 2025 Feb;90:102963. doi: 10.1016/j.conb.2024.102963. Epub 2025 Jan 6. Curr Opin Neurobiol. 2025. PMID: 39765206 Free PMC article. Review.
-
Associations between appetite loss and clinical features as well as inflammatory cytokines in adolescents with major depressive disorder.Front Psychiatry. 2025 May 14;16:1583060. doi: 10.3389/fpsyt.2025.1583060. eCollection 2025. Front Psychiatry. 2025. PMID: 40438334 Free PMC article.
-
Circulating lung cancer exosomes damage the niche of intestinal stem cells.Transl Lung Cancer Res. 2025 Mar 31;14(3):718-735. doi: 10.21037/tlcr-24-758. Epub 2025 Mar 10. Transl Lung Cancer Res. 2025. PMID: 40248740 Free PMC article.
-
Cancer neuroscience at the brain-body interface.Genes Dev. 2024 Oct 16;38(17-20):787-792. doi: 10.1101/gad.352288.124. Genes Dev. 2024. PMID: 39362778 Free PMC article. Review.
-
Refractory Nausea and Vomiting Due to Central Nervous System Injury: A Focused Review.Life (Basel). 2025 Jun 27;15(7):1021. doi: 10.3390/life15071021. Life (Basel). 2025. PMID: 40724523 Free PMC article. Review.
References
-
- Biswas AK, Acharyya S. Cancer-associated cachexia: a systemic consequence of cancer progression. Annu. Rev. Cancer Biol. 2020;4:391–411. doi: 10.1146/annurev-cancerbio-030419-033642. - DOI
MeSH terms
Substances
Grants and funding
- R01 DA050374/DA/NIDA NIH HHS/United States
- R01 CA188134/CA/NCI NIH HHS/United States
- Cold Spring Harbor Laboratory and Northwell Health Affiliation/Cold Spring Harbor Laboratory (CSHL)
- R01 CA190092/CA/NCI NIH HHS/United States
- U01 CA224013/CA/NCI NIH HHS/United States
- R01 CA229699/CA/NCI NIH HHS/United States
- F31 MH124365/MH/NIMH NIH HHS/United States
- R01 NS104944/NS/NINDS NIH HHS/United States
- U01 CA210240/CA/NCI NIH HHS/United States
- OT2 CA278690/CA/NCI NIH HHS/United States
- R01 MH101214/MH/NIMH NIH HHS/United States
- CGCATF-2021/100026/CRUK_/Cancer Research UK/United Kingdom
- R50 CA211506/CA/NCI NIH HHS/United States
- R01 MH108924/MH/NIMH NIH HHS/United States
- P30 CA045508/CA/NCI NIH HHS/United States
- R01 MH113628/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

