A persistent variant telomere sequence in a human pedigree
- PMID: 38824190
- PMCID: PMC11144197
- DOI: 10.1038/s41467-024-49072-9
A persistent variant telomere sequence in a human pedigree
Abstract
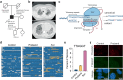
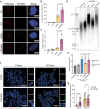
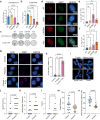
The telomere sequence, TTAGGG, is conserved across all vertebrates and plays an essential role in suppressing the DNA damage response by binding a set of proteins termed shelterin. Changes in the telomere sequence impair shelterin binding, initiate a DNA damage response, and are toxic to cells. Here we identify a family with a variant in the telomere template sequence of telomerase, the enzyme responsible for telomere elongation, that led to a non-canonical telomere sequence. The variant is inherited across at least one generation and one family member reports no significant medical concerns despite ~9% of their telomeres converting to the novel sequence. The variant template disrupts telomerase repeat addition processivity and decreased the binding of the telomere-binding protein POT1. Despite these disruptions, the sequence is readily incorporated into cellular chromosomes. Incorporation of a variant sequence prevents POT1-mediated inhibition of telomerase suggesting that incorporation of a variant sequence may influence telomere addition. These findings demonstrate that telomeres can tolerate substantial degeneracy while remaining functional and provide insights as to how incorporation of a non-canonical telomere sequence might alter telomere length dynamics.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

