Preserving condensate structure and composition by lowering sequence complexity
- PMID: 38824391
- PMCID: PMC11267431
- DOI: 10.1016/j.bpj.2024.05.026
Preserving condensate structure and composition by lowering sequence complexity
Abstract
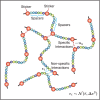
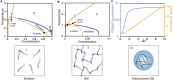
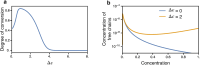
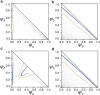
Biomolecular condensates play a vital role in organizing cellular chemistry. They selectively partition biomolecules, preventing unwanted cross talk and buffering against chemical noise. Intrinsically disordered proteins (IDPs) serve as primary components of these condensates due to their flexibility and ability to engage in multivalent interactions, leading to spontaneous aggregation. Theoretical advancements are critical at connecting IDP sequences with condensate emergent properties to establish the so-called molecular grammar. We proposed an extension to the stickers and spacers model, incorporating heterogeneous, nonspecific pairwise interactions between spacers alongside specific interactions among stickers. Our investigation revealed that although spacer interactions contribute to phase separation and co-condensation, their nonspecific nature leads to disorganized condensates. Specific sticker-sticker interactions drive the formation of condensates with well-defined networked structures and molecular composition. We discussed how evolutionary pressures might emerge to affect these interactions, leading to the prevalence of low-complexity domains in IDP sequences. These domains suppress spurious interactions and facilitate the formation of biologically meaningful condensates.
Copyright © 2024 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Authors declare that they have no competing interests.
Figures
Update of
-
Preserving condensate structure and composition by lowering sequence complexity.bioRxiv [Preprint]. 2023 Nov 29:2023.11.29.569249. doi: 10.1101/2023.11.29.569249. bioRxiv. 2023. Update in: Biophys J. 2024 Jul 2;123(13):1815-1826. doi: 10.1016/j.bpj.2024.05.026. PMID: 38076908 Free PMC article. Updated. Preprint.
Similar articles
-
Preserving condensate structure and composition by lowering sequence complexity.bioRxiv [Preprint]. 2023 Nov 29:2023.11.29.569249. doi: 10.1101/2023.11.29.569249. bioRxiv. 2023. Update in: Biophys J. 2024 Jul 2;123(13):1815-1826. doi: 10.1016/j.bpj.2024.05.026. PMID: 38076908 Free PMC article. Updated. Preprint.
-
Uncovering protein conformational dynamics within two-component viral biomolecular condensates.Protein Sci. 2025 Jul;34(7):e70181. doi: 10.1002/pro.70181. Protein Sci. 2025. PMID: 40521608 Free PMC article.
-
Disordered Regions of Condensate-promoting Proteins Have Distinct Molecular Signatures Associated with Cellular Function.J Mol Biol. 2025 Mar 1;437(5):168953. doi: 10.1016/j.jmb.2025.168953. Epub 2025 Jan 16. J Mol Biol. 2025. PMID: 39826710
-
A story of two kingdoms: unravelling the intricacies of protein phase separation in plants and animals.Crit Rev Biotechnol. 2025 Aug;45(5):1019-1039. doi: 10.1080/07388551.2024.2425989. Epub 2024 Nov 26. Crit Rev Biotechnol. 2025. PMID: 39592156 Review.
-
RNA-associated nuclear condensates: Where the nucleus keeps its RNAs in check.Mol Cells. 2025 Aug;48(8):100240. doi: 10.1016/j.mocell.2025.100240. Epub 2025 Jun 15. Mol Cells. 2025. PMID: 40527409 Free PMC article. Review.
Cited by
-
Survey of the Aβ-peptide structural diversity: molecular dynamics approaches.Biophys Rev. 2024 Nov 20;16(6):701-722. doi: 10.1007/s12551-024-01253-y. eCollection 2024 Dec. Biophys Rev. 2024. PMID: 39830132 Review.
References
-
- Ginell G.M., Holehouse A.S. Springer US; 2022. An Introduction to the Stickers-And-Spacers Framework as Applied to Biomolecular Condensates; pp. 95–116. - PubMed
-
- Berry J., Brangwynne C.P., Haataja M. Physical principles of intracellular organization via active and passive phase transitions. Rep. Prog. Phys. 2018;81 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

