A gain-of-function mutation in BnaIAA13 disrupts vascular tissue and lateral root development in Brassica napus
- PMID: 38824403
- PMCID: PMC11427839
- DOI: 10.1093/jxb/erae245
A gain-of-function mutation in BnaIAA13 disrupts vascular tissue and lateral root development in Brassica napus
Erratum in
-
Correction to: A gain-of-function mutation in BnaIAA13 disrupts vascular tissue and lateral root development in Brassica napus.J Exp Bot. 2024 Sep 27;75(18):6006. doi: 10.1093/jxb/erae345. J Exp Bot. 2024. PMID: 39152712 Free PMC article. No abstract available.
Abstract
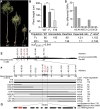
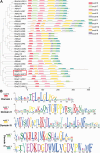
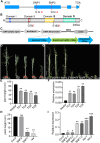
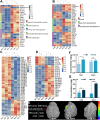
Rapeseed (Brassica napus) is an important oilseed crop worldwide. Plant vascular tissues are responsible for long-distance transport of water and nutrients and for providing mechanical support. The lateral roots absorb water and nutrients. The genetic basis of vascular tissue and lateral root development in rapeseed remains unknown. This study characterized an ethyl methanesulfonate-mutagenized rapeseed mutant, T16, which showed dwarf stature, reduced lateral roots, and leaf wilting. SEM observations showed that the internode cells were shortened. Observations of tissue sections revealed defects in vascular bundle development in the stems and petioles. Genetic analysis revealed that the phenotypes of T16 were controlled by a single semi-dominant nuclear gene. Map-based cloning and genetic complementarity identified BnaA03.IAA13 as the functional gene; a G-to-A mutation in the second exon changed glycine at position 79 to glutamic acid, disrupting the conserved degron motif VGWPP. Transcriptome analysis in roots and stems showed that auxin and cytokinin signaling pathways were disordered in T16. Evolutionary analysis showed that AUXIN/INDOLE-3-ACETIC ACID is conserved during plant evolution. The heterozygote of T16 showed significantly reduced plant height while maintaining other agronomic traits. Our findings provide novel insights into the regulatory mechanisms of vascular tissue and lateral root development, and offer a new germplasm resource for rapeseed breeding.
Keywords: BnaA03.IAA13; Brassica napus; lateral root; map-based cloning; plant height; vascular tissue.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
An auxin signaling gene BnaA3.IAA7 contributes to improved plant architecture and yield heterosis in rapeseed.New Phytol. 2019 Apr;222(2):837-851. doi: 10.1111/nph.15632. Epub 2019 Jan 5. New Phytol. 2019. PMID: 30536633
-
Integrated genetic mapping and transcriptome analysis reveal the BnaA03.IAA7 protein regulates plant architecture and gibberellin signaling in Brassica napus L.Theor Appl Genet. 2022 Oct;135(10):3497-3510. doi: 10.1007/s00122-022-04196-8. Epub 2022 Aug 12. Theor Appl Genet. 2022. PMID: 35962210
-
A Gain-of-Function Mutant of IAA7 Inhibits Stem Elongation by Transcriptional Repression of EXPA5 Genes in Brassica napus.Int J Mol Sci. 2021 Aug 21;22(16):9018. doi: 10.3390/ijms22169018. Int J Mol Sci. 2021. PMID: 34445724 Free PMC article.
-
Initiation of vascular development.Physiol Plant. 2014 Jun;151(2):142-6. doi: 10.1111/ppl.12111. Epub 2013 Oct 21. Physiol Plant. 2014. PMID: 24111590 Review.
-
The Use of Genetic and Gene Technologies in Shaping Modern Rapeseed Cultivars (Brassica napus L.).Genes (Basel). 2020 Sep 30;11(10):1161. doi: 10.3390/genes11101161. Genes (Basel). 2020. PMID: 33008008 Free PMC article. Review.
References
-
- Bishopp A, Lehesranta S, Vatén A, et al.. 2011. Phloem-transported cytokinin regulates polar auxin transport and maintains vascular pattern in the root meristem. Current Biology 21, 927–932. - PubMed
-
- Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett MJ.. 2003. Dissecting Arabidopsis lateral root development. Trends in Plant Science 8, 165–171. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

