Stepped Palliative Care for Patients With Advanced Lung Cancer: A Randomized Clinical Trial
- PMID: 38824442
- PMCID: PMC11145511
- DOI: 10.1001/jama.2024.10398
Stepped Palliative Care for Patients With Advanced Lung Cancer: A Randomized Clinical Trial
Abstract
Importance: Despite the evidence for early palliative care improving outcomes, it has not been widely implemented in part due to palliative care workforce limitations.
Objective: To evaluate a stepped-care model to deliver less resource-intensive and more patient-centered palliative care for patients with advanced cancer.
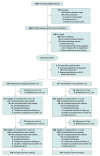
Design, setting, and participants: Randomized, nonblinded, noninferiority trial of stepped vs early palliative care conducted between February 12, 2018, and December 15, 2022, at 3 academic medical centers in Boston, Massachusetts, Philadelphia, Pennsylvania, and Durham, North Carolina, among 507 patients who had been diagnosed with advanced lung cancer within the past 12 weeks.
Intervention: Step 1 of the intervention was an initial palliative care visit within 4 weeks of enrollment and subsequent visits only at the time of a change in cancer treatment or after a hospitalization. During step 1, patients completed a measure of quality of life (QOL; Functional Assessment of Cancer Therapy-Lung [FACT-L]; range, 0-136, with higher scores indicating better QOL) every 6 weeks, and those with a 10-point or greater decrease from baseline were stepped up to meet with the palliative care clinician every 4 weeks (intervention step 2). Patients assigned to early palliative care had palliative care visits every 4 weeks after enrollment.
Main outcomes and measures: Noninferiority (margin = -4.5) of the effect of stepped vs early palliative care on patient-reported QOL on the FACT-L at week 24.
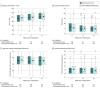
Results: The sample (n = 507) mostly included patients with advanced non-small cell lung cancer (78.3%; mean age, 66.5 years; 51.4% female; 84.6% White). The mean number of palliative care visits by week 24 was 2.4 for stepped palliative care and 4.7 for early palliative care (adjusted mean difference, -2.3; P < .001). FACT-L scores at week 24 for the stepped palliative care group were noninferior to scores among those receiving early palliative care (adjusted FACT-L mean score, 100.6 vs 97.8, respectively; difference, 2.9; lower 1-sided 95% confidence limit, -0.1; P < .001 for noninferiority). Although the rate of end-of-life care communication was also noninferior between groups, noninferiority was not demonstrated for days in hospice (adjusted mean, 19.5 with stepped palliative care vs 34.6 with early palliative care; P = .91).
Conclusions and relevance: A stepped-care model, with palliative care visits occurring only at key points in patients' cancer trajectories and using a decrement in QOL to trigger more intensive palliative care exposure, resulted in fewer palliative care visits without diminishing the benefits for patients' QOL. While stepped palliative care was associated with fewer days in hospice, it is a more scalable way to deliver early palliative care to enhance patient-reported outcomes.
Trial registration: ClinicalTrials.gov Identifier: NCT03337399.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

