PI3Kγ inhibition combined with DNA vaccination unleashes a B-cell-dependent antitumor immunity that hampers pancreatic cancer
- PMID: 38824552
- PMCID: PMC11143614
- DOI: 10.1186/s13046-024-03080-1
PI3Kγ inhibition combined with DNA vaccination unleashes a B-cell-dependent antitumor immunity that hampers pancreatic cancer
Abstract
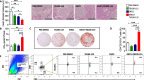
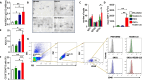
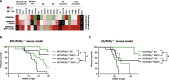
Phosphoinositide-3-kinase γ (PI3Kγ) plays a critical role in pancreatic ductal adenocarcinoma (PDA) by driving the recruitment of myeloid-derived suppressor cells (MDSC) into tumor tissues, leading to tumor growth and metastasis. MDSC also impair the efficacy of immunotherapy. In this study we verify the hypothesis that MDSC targeting, via PI3Kγ inhibition, synergizes with α-enolase (ENO1) DNA vaccination in counteracting tumor growth.Mice that received ENO1 vaccination followed by PI3Kγ inhibition had significantly smaller tumors compared to those treated with ENO1 alone or the control group, and correlated with i) increased circulating anti-ENO1 specific IgG and IFNγ secretion by T cells, ii) increased tumor infiltration of CD8+ T cells and M1-like macrophages, as well as up-modulation of T cell activation and M1-like related transcripts, iii) decreased infiltration of Treg FoxP3+ T cells, endothelial cells and pericytes, and down-modulation of the stromal compartment and T cell exhaustion gene transcription, iv) reduction of mature and neo-formed vessels, v) increased follicular helper T cell activation and vi) increased "antigen spreading", as many other tumor-associated antigens were recognized by IgG2c "cytotoxic" antibodies. PDA mouse models genetically devoid of PI3Kγ showed an increased survival and a pattern of transcripts in the tumor area similar to that of pharmacologically-inhibited PI3Kγ-proficient mice. Notably, tumor reduction was abrogated in ENO1 + PI3Kγ inhibition-treated mice in which B cells were depleted.These data highlight a novel role of PI3Kγ in B cell-dependent immunity, suggesting that PI3Kγ depletion strengthens the anti-tumor response elicited by the ENO1 DNA vaccine.
© 2024. The Author(s).
Conflict of interest statement
EH is co-funder and board member of Kither Biotech, JAV is a consultant for Infinity Pharmaceuticals and a shareholder in Impact Biosciences and AlphaBeta Therapeutics.
Figures




Similar articles
-
Macrophage PI3Kγ Drives Pancreatic Ductal Adenocarcinoma Progression.Cancer Discov. 2016 Aug;6(8):870-85. doi: 10.1158/2159-8290.CD-15-1346. Epub 2016 May 13. Cancer Discov. 2016. PMID: 27179037 Free PMC article.
-
Induction of macrophage efferocytosis in pancreatic cancer via PI3Kγ inhibition and radiotherapy promotes tumour control.Gut. 2025 Apr 7;74(5):825-839. doi: 10.1136/gutjnl-2024-333492. Gut. 2025. PMID: 39788719 Free PMC article.
-
Vaccination with ENO1 DNA prolongs survival of genetically engineered mice with pancreatic cancer.Gastroenterology. 2013 May;144(5):1098-106. doi: 10.1053/j.gastro.2013.01.020. Epub 2013 Jan 16. Gastroenterology. 2013. PMID: 23333712
-
Targeting PI3K-gamma in myeloid driven tumour immune suppression: a systematic review and meta-analysis of the preclinical literature.Cancer Immunol Immunother. 2024 Aug 6;73(10):204. doi: 10.1007/s00262-024-03779-2. Cancer Immunol Immunother. 2024. PMID: 39105848 Free PMC article.
-
Next Generation Immunotherapy for Pancreatic Cancer: DNA Vaccination is Seeking New Combo Partners.Cancers (Basel). 2018 Feb 16;10(2):51. doi: 10.3390/cancers10020051. Cancers (Basel). 2018. PMID: 29462900 Free PMC article. Review.
Cited by
-
In pancreatic cancer patients, chemotherapy reshapes the gene expression profile and antigen receptor repertoire of T lymphocytes and enhances their effector response to tumor-associated antigens.Front Immunol. 2024 Aug 8;15:1427424. doi: 10.3389/fimmu.2024.1427424. eCollection 2024. Front Immunol. 2024. PMID: 39176093 Free PMC article.
-
CD8+ T cell exhaustion and its regulatory mechanisms in the tumor microenvironment: key to the success of immunotherapy.Front Immunol. 2024 Sep 20;15:1476904. doi: 10.3389/fimmu.2024.1476904. eCollection 2024. Front Immunol. 2024. PMID: 39372416 Free PMC article. Review.
-
The absence of IL17A favours cytotoxic cell function and improves antigen-specific immunotherapies in pancreatic adenocarcinoma.Clin Transl Med. 2025 Aug;15(8):e70442. doi: 10.1002/ctm2.70442. Clin Transl Med. 2025. PMID: 40831074 Free PMC article.
-
Invasion and metastasis in cancer: molecular insights and therapeutic targets.Signal Transduct Target Ther. 2025 Feb 21;10(1):57. doi: 10.1038/s41392-025-02148-4. Signal Transduct Target Ther. 2025. PMID: 39979279 Free PMC article. Review.
-
OSBPL3 modulates the immunosuppressive microenvironment and predicts therapeutic outcomes in pancreatic cancer.Biol Direct. 2025 Jan 9;20(1):5. doi: 10.1186/s13062-025-00596-0. Biol Direct. 2025. PMID: 39789613 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

