A systematic review of assisted and third-party reproduction guidelines regarding management and care of donors
- PMID: 38824591
- PMCID: PMC11143578
- DOI: 10.1186/s12978-024-01804-2
A systematic review of assisted and third-party reproduction guidelines regarding management and care of donors
Abstract
Background: Gamete and embryo donors face complex challenges affecting their health and quality of life. Healthcare providers need access to well-structured, evidence-based, and needs-based guidance to care for gamete and embryo donors. Therefore, this systematic review aimed to synthesize current assisted and third-party reproduction guidelines regarding management and care of donors.
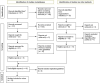
Methods: The databases of ISI, PubMed, Scopus, and websites of organizations related to the assisted reproduction were searched using the keywords of "third party reproduction", "gamete donation", "embryo donation", "guidelines", "committee opinion", and "best practice", without time limit up to July 2023. All the clinical or ethical guidelines and best practice statements regarding management and care for gamete and embryo donors written in the English language were included in the study. Quality assessment was carried using AGREE II tool. Included documents were reviewed and extracted data were narratively synthesized.
Results: In this systematic review 14 related documents were reviewed of which eight were guidelines, three were practice codes and three were committee opinions. Five documents were developed in the United States, three in Canada, two in the United Kingdom, one in Australia, and one in Australia and New Zealand. Also, two guidelines developed by the European Society of Human Reproduction and Embryology were found. Management and care provided for donors were classified into four categories including screening, counseling, information provision, and ethical considerations.
Conclusion: While the current guidelines include some recommendations regarding the management and care of gamete/embryo donors in screening, counseling, information provision, and ethical considerations, nevertheless some shortcomings need to be addressed including donors' psychosocial needs, long-term effects of donation, donors' follow-up cares, and legal and human rights aspects of donation. Therefore, it is needed to conduct robust and well-designed research studies to fill the knowledge gap about gamete and embryo donors' needs, to inform current practices by developing evidence-based guidelines.
Keywords: Egg donor; Embryo donor; Gamete donor; Guidelines; Reproductive donation; Sperm donor; Third-party reproduction.
Plain language summary
Gamete and embryo donors face complex challenges affecting their health and quality of life. To manage these challenges, healthcare providers need guidelines that are based on evidence and donors’ real needs. In order to develop a comprehensive guideline that meets the needs of donors; it is important to review the current guidelines. So, in this study we reviewed the current assisted and third-party reproduction guidelines regarding management and care of donors. We searched databases and relevant websites and found 14 related documents. The main topics recommended for management and care of donors in these guidelines included screening, counseling, information provision, and ethical considerations. We recognized that some of donors’ needs are neglected in these documents including donors’ psychosocial needs, long-term effects of donation on donors, their follow-up cares, and legal and human rights aspects of donation. Therefore, there is need for further research to develop guidelines based on donors’ unmet needs.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Ethical Issues in Sperm, Egg and Embryo Donation: Islamic Shia Perspectives.HEC Forum. 2024 Jun;36(2):167-185. doi: 10.1007/s10730-022-09498-4. Epub 2022 Nov 12. HEC Forum. 2024. PMID: 36371516 Review.
-
Gamete and embryo donation and surrogacy in australia: the social context and regulatory framework.Int J Fertil Steril. 2011 Jan;4(4):176-83. Epub 2011 Feb 20. Int J Fertil Steril. 2011. PMID: 24851179 Free PMC article.
-
Survey report of gamete donors' and recipients' preferences regarding disclosure of third party reproduction outcomes and genetic risk information.J Obstet Gynaecol Res. 2011 Apr;37(4):292-9. doi: 10.1111/j.1447-0756.2010.01333.x. Epub 2011 Feb 23. J Obstet Gynaecol Res. 2011. PMID: 21349122
-
Good practice recommendations for information provision for those involved in reproductive donation†.Hum Reprod Open. 2022 Feb 16;2022(1):hoac001. doi: 10.1093/hropen/hoac001. eCollection 2022. Hum Reprod Open. 2022. PMID: 35178481 Free PMC article.
-
Ethics of oocyte banking for third-party assisted reproduction: a systematic review.Hum Reprod Update. 2018 Sep 1;24(5):615-635. doi: 10.1093/humupd/dmy016. Hum Reprod Update. 2018. PMID: 29762669
References
-
- American Society for Reproductive Medicine. Gamete (Eggs And Sperm) And Embryo Donation. 2014. https://www.reproductivefacts.org/news-and-publications/patient-fact-she.... Cited 2021 Oct 7.
-
- European Society of Human Reproduction and Embryology (ESHRE). Information provision in donation, Good practice recommendations for information provision for those using and participating in reproductive donation, Guidelines under development. 2021. https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Guidelines-in-devel.... Cited 2022 Jan 7.
-
- Richards M, Pennings G, Appleby JB. Reproductive Donation: Practice, Policy and Bioethics. First edition. Cambridge: Cambridge University Press; 2012.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous