Cost-effectiveness of eculizumab and efgartigimod for the treatment of anti-acetylcholine receptor antibody-positive generalized myasthenia gravis
- PMID: 38824625
- PMCID: PMC11144987
- DOI: 10.18553/jmcp.2024.30.6.517
Cost-effectiveness of eculizumab and efgartigimod for the treatment of anti-acetylcholine receptor antibody-positive generalized myasthenia gravis
Abstract
Background: Eculizumab and efgartigimod were approved to treat anti-acetylcholine receptor antibody-positive generalized myasthenia gravis (anti-AChR Ab-positive gMG). These relatively new biological treatments provide a more rapid onset of action and improved efficacy compared with conventional immunosuppressive treatments, but at a higher cost.
Objective: To assess the cost-effectiveness of eculizumab and, separately, efgartigimod, each added to conventional therapy vs conventional therapy alone, among patients with refractory anti-AChR Ab-positive gMG and those with anti-AChR Ab-positive gMG, respectively.
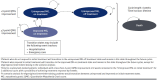
Methods: A Markov model with 4 health states was developed, evaluating costs and utility with a 4-week cycle length and lifetime time horizon from a health care system perspective and a modified societal perspective including productivity losses from patients and caregiver burden. Model inputs were informed by key clinical trials and relevant publications identified from targeted literature reviews, and drug costs were identified from Micromedex Red Book. Costs and outcomes were discounted at 3% per year. Incremental cost-effectiveness ratios (ICERs; cost per quality-adjusted life-year [QALY] gained) were calculated for each comparison.
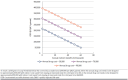
Results: Among the corresponding populations, lifetime costs and QALYs, respectively, for eculizumab were $5,515,000 and 11.85, and for conventional therapy, $308,000 and 10.29, resulting in an ICER of $3,338,000/QALY gained. For efgartigimod, lifetime costs and QALYs, respectively, were $6,773,000 and 13.22, and for conventional therapy, $322,000 and 9.98, yielding an ICER of $1,987,000/QALY gained. After applying indirect costs in a modified societal perspective, the ICERs were reduced to $3,310,000/QALY gained for eculizumab and $1,959,000/QALY gained for efgartigimod.
Conclusions: Eculizumab and efgartigimod are rapidly acting and effective treatments for myasthenia gravis. However, at their current price, both therapies greatly exceeded common cost-effectiveness thresholds, likely limiting patient access to these therapies.
Figures
References
-
- Howard J Jr. Myasthenia gravis clinical overview. 2015. Accessed May 5, 2021. https://myasthenia.org/Professionals/Clinical-Overview-of-MG
-
- Harris L, Graham S, MacLachlan S, Exuzides A, Jacob S. Healthcare resource utilization by patients with treatment-refractory myasthenia gravis in England. J Med Econ. 2019;22(7):691-7. doi:10.1080/13696998.2019.1592180 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical