The SLC6A18 Transporter Is Most Likely a Na-Dependent Glycine/Urea Antiporter Responsible for Urea Secretion in the Proximal Straight Tubule: Influence of This Urea Secretion on Glomerular Filtration Rate
- PMID: 38824912
- PMCID: PMC11651341
- DOI: 10.1159/000539602
The SLC6A18 Transporter Is Most Likely a Na-Dependent Glycine/Urea Antiporter Responsible for Urea Secretion in the Proximal Straight Tubule: Influence of This Urea Secretion on Glomerular Filtration Rate
Abstract
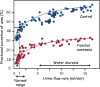
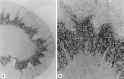
Background: Urea is the major end-product of protein metabolism in mammals. In carnivores and omnivores, a large load of urea is excreted daily in urine, with a concentration that is 30-100 times above that in plasma. This is important for the sake of water economy. Too little attention has been given to the existence of energy-dependent urea transport that plays an important role in this concentrating activity.
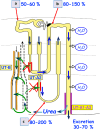
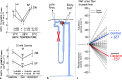
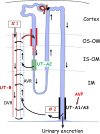
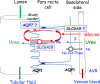
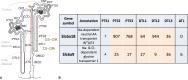
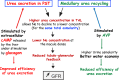
Summary: This review first presents functional evidence for an energy-dependent urea secretion that occurs exclusively in the straight part of the proximal tubule (PST). Second, it proposes a candidate transmembrane transporter responsible for this urea secretion in the PST. SLC6A18 is expressed exclusively in the PST and has been identified as a glycine transporter, based on findings in SLC6A18 knockout mice. We propose that it is actually a glycine/urea antiport, secreting urea into the lumen in exchange for glycine and Na. Glycine is most likely recycled back into the cell via a transporter located in the brush border. Urea secretion in the PST modifies the composition of the tubular fluid in the thick ascending limb and, thus, contributes, indirectly, to influence the "signal" at the macula densa that plays a crucial role in the regulation of the glomerular filtration rate (GFR) by the tubulo-glomerular feedback.
Key messages: Taking into account this secondary active secretion of urea in the mammalian kidney provides a new understanding of the influence of protein intake on GFR, of the regulation of urea excretion, and of the urine-concentrating mechanism.
Keywords: Familial azotemia; Fractional excretion; Glomerular filtration rate; Glycine; Pars recta.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
No conflict of interest for any of the authors.
Figures




References
-
- Bankir L, Roussel R, Bouby N. Protein- and diabetes-induced glomerular hyperfiltration: role of glucagon, vasopressin, and urea. Am J Physiol Renal Physiol. 2015;309(1):F2–23. - PubMed
-
- Bankir L, Ahloulay M, Bouby N, Trinh-Trang-Tan MM, Machet F, Lacour B, et al. . Is the process of urinary urea concentration responsible for a high glomerular filtration rate? J Am Soc Nephrol. 1993;4(5):1091–103. - PubMed
-
- Bankir L, Bouby N, Trinh-Trang-Tan MM, Ahloulay M, Promeneur D. Direct and indirect cost of urea excretion. Kidney Int. 1996;49(6):1598–607. - PubMed
-
- Gamble JL, McKhann CF, Butler AM, Tuthill E. An economy of water in renal function referable to urea. Am J Physiol. 1934;109(1):139–54.
-
- Fenton RA, Chou CL, Sowersby H, Smith CP, Knepper MA. Gamble’s “economy of water” revisited: studies in urea transporter knockout mice. Am J Physiol Renal Physiol. 2006;291(1):F148–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

