Pharmacokinetic-Guided Hydroxyurea to Reduce Transfusions in Ugandan Children with Sickle Cell Anemia: Study Design of the Alternative Dosing And Prevention of Transfusions Trial
- PMID: 38824918
- PMCID: PMC11617603
- DOI: 10.1159/000539541
Pharmacokinetic-Guided Hydroxyurea to Reduce Transfusions in Ugandan Children with Sickle Cell Anemia: Study Design of the Alternative Dosing And Prevention of Transfusions Trial
Abstract
Introduction: People with sickle cell anemia (SCA) may require frequent blood transfusions to treat acute and chronic complications. Hydroxyurea is a life-saving treatment for SCA that could also decrease the need for blood transfusions. Inadequate medication access and challenges in dose optimization limit the widespread use of hydroxyurea in Africa. If feasible, pharmacokinetic (PK) dosing might improve dose determination to minimize toxicities and maximize clinical benefits. The Alternative Dosing And Prevention of Transfusions (ADAPT, NCT05662098) trial will analyze the impact of hydroxyurea on transfusion rate and serve as a pilot study to evaluate the feasibility of PK-guided hydroxyurea dosing in Uganda.
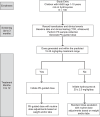
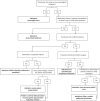
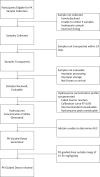
Methods: Herein we describe the rationale and design of ADAPT, a prospective cohort study of ∼100 children with SCA in Jinja, Uganda. The primary hypothesis is that hydroxyurea will decrease blood transfusion use by ≥ 50%, comparing the transfusion incidence rate ratio between a 3-month pretreatment and a 12-month treatment period. A key secondary hypothesis is that our PK-dosing approach will generate a suitable hydroxyurea dose for ≥80% of participants. Every ADAPT participant will undergo hydroxyurea PK testing, and if a dose is generated within 15-35 mg/kg/day, participants will start on their individualized dose. If not, they will start on a default dose of 20 mg/kg/day. Hydroxyurea dose optimization will occur with periodic dose adjustments.
Conclusion: Overall, demonstrating the reduction in blood transfusion utilization with hydroxyurea treatment would provide leverage to increase hydroxyurea access, and PK-guided hydroxyurea dosing should optimize the safe and effective treatment of SCA across sub-Saharan Africa.
Introduction: People with sickle cell anemia (SCA) may require frequent blood transfusions to treat acute and chronic complications. Hydroxyurea is a life-saving treatment for SCA that could also decrease the need for blood transfusions. Inadequate medication access and challenges in dose optimization limit the widespread use of hydroxyurea in Africa. If feasible, pharmacokinetic (PK) dosing might improve dose determination to minimize toxicities and maximize clinical benefits. The Alternative Dosing And Prevention of Transfusions (ADAPT, NCT05662098) trial will analyze the impact of hydroxyurea on transfusion rate and serve as a pilot study to evaluate the feasibility of PK-guided hydroxyurea dosing in Uganda.
Methods: Herein we describe the rationale and design of ADAPT, a prospective cohort study of ∼100 children with SCA in Jinja, Uganda. The primary hypothesis is that hydroxyurea will decrease blood transfusion use by ≥ 50%, comparing the transfusion incidence rate ratio between a 3-month pretreatment and a 12-month treatment period. A key secondary hypothesis is that our PK-dosing approach will generate a suitable hydroxyurea dose for ≥80% of participants. Every ADAPT participant will undergo hydroxyurea PK testing, and if a dose is generated within 15-35 mg/kg/day, participants will start on their individualized dose. If not, they will start on a default dose of 20 mg/kg/day. Hydroxyurea dose optimization will occur with periodic dose adjustments.
Conclusion: Overall, demonstrating the reduction in blood transfusion utilization with hydroxyurea treatment would provide leverage to increase hydroxyurea access, and PK-guided hydroxyurea dosing should optimize the safe and effective treatment of SCA across sub-Saharan Africa.
Keywords: Hydroxyurea; Personalized medicine; Pharmacokinetics; Sickle cell anemia; Transfusion medicine.
© 2024 S. Karger AG, Basel.
Conflict of interest statement
R.E.W. serves as a Medical Advisor to Nova Laboratories. The other authors have no conflicts of interests to disclose.
Figures

Similar articles
-
The feasibility of pharmacokinetic-based dosing of hydroxyurea for children with sickle cell anaemia in Uganda: Baseline results of the alternative dosing and prevention of transfusions trial.Br J Clin Pharmacol. 2025 Jun;91(6):1865-1872. doi: 10.1111/bcp.70071. Br J Clin Pharmacol. 2025. PMID: 40441700 Free PMC article. Clinical Trial.
-
Reducing transfusion utilization for children with sickle cell anemia in sub-Saharan Africa with hydroxyurea: Analysis from the phase I/II REACH trial.Am J Hematol. 2024 Apr;99(4):625-632. doi: 10.1002/ajh.27244. Epub 2024 Feb 8. Am J Hematol. 2024. PMID: 38332651 Free PMC article. Clinical Trial.
-
Robust clinical and laboratory response to hydroxyurea using pharmacokinetically guided dosing for young children with sickle cell anemia.Am J Hematol. 2019 Aug;94(8):871-879. doi: 10.1002/ajh.25510. Epub 2019 Jun 12. Am J Hematol. 2019. PMID: 31106898 Free PMC article.
-
Blood transfusion for preventing primary and secondary stroke in people with sickle cell disease.Cochrane Database Syst Rev. 2020 Jul 27;7(7):CD003146. doi: 10.1002/14651858.CD003146.pub4. Cochrane Database Syst Rev. 2020. PMID: 32716555 Free PMC article.
-
Interventions for preventing silent cerebral infarcts in people with sickle cell disease.Cochrane Database Syst Rev. 2020 Apr 6;4(4):CD012389. doi: 10.1002/14651858.CD012389.pub3. Cochrane Database Syst Rev. 2020. PMID: 32250453 Free PMC article.
Cited by
-
The feasibility of pharmacokinetic-based dosing of hydroxyurea for children with sickle cell anaemia in Uganda: Baseline results of the alternative dosing and prevention of transfusions trial.Br J Clin Pharmacol. 2025 Jun;91(6):1865-1872. doi: 10.1111/bcp.70071. Br J Clin Pharmacol. 2025. PMID: 40441700 Free PMC article. Clinical Trial.
-
The feasibility of pharmacokinetic-based dosing of hydroxyurea for children with sickle cell anaemia in Uganda: Baseline results of the alternative dosing and prevention of transfusions trial.Br J Clin Pharmacol. 2025 Apr 13;91(6):1865-72. doi: 10.1002/bcp.70071. Online ahead of print. Br J Clin Pharmacol. 2025. PMID: 40222814 Free PMC article.
References
-
- World Health Organization . Fifty-ninth world health assembly. WHA5920: sickle cell anemia; 2006.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

