Efficacy and adverse events of L-Asparaginase administration as a first-line treatment for feline large-cell gastrointestinal lymphoma
- PMID: 38825481
- PMCID: PMC11251808
- DOI: 10.1292/jvms.23-0453
Efficacy and adverse events of L-Asparaginase administration as a first-line treatment for feline large-cell gastrointestinal lymphoma
Abstract
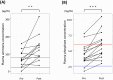
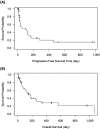
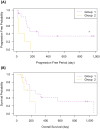
L-Asparaginase (L-Asp) is often used to induce remission in feline large-cell gastrointestinal lymphoma (LCGIL). However, no study has evaluated the efficacy and adverse events following the initial use of this drug as a first-line treatment in feline LCGIL. We retrospectively reviewed medical records of cats with LCGIL treated with L-Asp to induce remission. This study included 43 cats. The response rate (RR) after the first administration of L-Asp was 37.2% (Complete remission: 7.0%, partial remission: 30.2%). RR was significantly higher in cases with primary gastric lesions (64.3%) than in those with primary intestinal lesions (24.1%) (P=0.018), and it was also higher in cases without anemia (57.1%) than those with anemia (15.0%) (P=0.009). The most common adverse event was hyperammonemia, which occurred in 10 of 12 cases where we could compare plasma ammonia concentrations before and after the first dose of L-Asp. Plasma phosphate concentrations were also significantly increased (P<0.001) within 24 hr after the first dose. Decreased appetite, vomiting, and diarrhea were also observed in five, three, and seven cases, respectively, and Grade 3 or higher gastrointestinal signs were observed as adverse events in three cases. The median overall survival of all cats was 150 days (range, 5-1,065 days), and the median progression-free survival was 104 days (range, 2-978 days). In conclusion, L-Asp was effective to induce remission, and severe adverse events were uncommon in feline LCGIL.
Keywords: ammonia; cat; chemotherapy; phosphate; toxicity.
Conflict of interest statement
The authors have nothing to disclose.
Figures
Similar articles
-
A retrospective evaluation of lomustine (CeeNU) in 32 treatment naïve cats with intermediate to large cell gastrointestinal lymphoma (2006-2013).Vet Comp Oncol. 2017 Sep;15(3):1019-1028. doi: 10.1111/vco.12243. Epub 2016 Jun 9. Vet Comp Oncol. 2017. PMID: 27277825
-
Neutropenia associated with vincristine and L-asparaginase induction chemotherapy for canine lymphoma.J Vet Intern Med. 2002 Sep-Oct;16(5):570-5. doi: 10.1892/0891-6640(2002)016<0570:nawval>2.3.co;2. J Vet Intern Med. 2002. PMID: 12322708 Clinical Trial.
-
Effects of L-asparaginase on plasma amino acid profiles and tumor burden in cats with lymphoma.J Vet Intern Med. 2007 Jul-Aug;21(4):760-3. doi: 10.1892/0891-6640(2007)21[760:eolopa]2.0.co;2. J Vet Intern Med. 2007. PMID: 17708396 Clinical Trial.
-
Feline gastrointestinal lymphoma.Vet Clin North Am Small Anim Pract. 2003 Sep;33(5):1083-98, vii. doi: 10.1016/s0195-5616(03)00054-8. Vet Clin North Am Small Anim Pract. 2003. PMID: 14552162 Review.
-
Principles of treatment for feline lymphoma.Clin Tech Small Anim Pract. 2003 May;18(2):98-102. doi: 10.1053/svms.2003.36623. Clin Tech Small Anim Pract. 2003. PMID: 12831069 Review.
Cited by
-
Histopathological patterns and immunophenotyping of feline lymphomas and incidence in Metropolitan Bangkok, Thailand.Vet World. 2024 Oct;17(10):2225-2234. doi: 10.14202/vetworld.2024.2225-2234. Epub 2024 Oct 4. Vet World. 2024. PMID: 39619931 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

