Antitumor efficacy and potential mechanism of FAP-targeted radioligand therapy combined with immune checkpoint blockade
- PMID: 38825657
- PMCID: PMC11144707
- DOI: 10.1038/s41392-024-01853-w
Antitumor efficacy and potential mechanism of FAP-targeted radioligand therapy combined with immune checkpoint blockade
Abstract
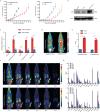
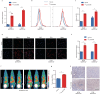
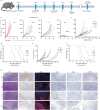
Radiotherapy combined with immune checkpoint blockade holds great promise for synergistic antitumor efficacy. Targeted radionuclide therapy delivers radiation directly to tumor sites. LNC1004 is a fibroblast activation protein (FAP)-targeting radiopharmaceutical, conjugated with the albumin binder Evans Blue, which has demonstrated enhanced tumor uptake and retention in previous preclinical and clinical studies. Herein, we demonstrate that 68Ga/177Lu-labeled LNC1004 exhibits increased uptake and prolonged retention in MC38/NIH3T3-FAP and CT26/NIH3T3-FAP tumor xenografts. Radionuclide therapy with 177Lu-LNC1004 induced a transient upregulation of PD-L1 expression in tumor cells. The combination of 177Lu-LNC1004 and anti-PD-L1 immunotherapy led to complete eradication of all tumors in MC38/NIH3T3-FAP tumor-bearing mice, with mice showing 100% tumor rejection upon rechallenge. Immunohistochemistry, single-cell RNA sequencing (scRNA-seq), and TCR sequencing revealed that combination therapy reprogrammed the tumor microenvironment in mice to foster antitumor immunity by suppressing malignant progression and increasing cell-to-cell communication, CD8+ T-cell activation and expansion, M1 macrophage counts, antitumor activity of neutrophils, and T-cell receptor diversity. A preliminary clinical study demonstrated that 177Lu-LNC1004 was well-tolerated and effective in patients with refractory cancers. Further, scRNA-seq of peripheral blood mononuclear cells underscored the importance of addressing immune evasion through immune checkpoint blockade treatment. This was emphasized by the observed increase in antigen processing and presentation juxtaposed with T cell inactivation. In conclusion, our data supported the efficacy of immunotherapy combined with 177Lu-LNC1004 for cancer patients with FAP-positive tumors.
© 2024. The Author(s).
Conflict of interest statement
X. Chen is a co-founder of the Yantai LNC Biotechnology. The remaining authors declare no competing interests.
Figures




References
-
- Chang JY, et al. Stereotactic ablative radiotherapy with or without immunotherapy for early-stage or isolated lung parenchymal recurrent node-negative non-small-cell lung cancer: an open-label, randomised, phase 2 trial. Lancet. 2023;402:871–881. doi: 10.1016/S0140-6736(23)01384-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 82071961/National Natural Science Foundation of China (National Science Foundation of China)
- 82272037/National Natural Science Foundation of China (National Science Foundation of China)
- NUHSRO/2023/008/NUSMed/TCE/LOA/National University of Singapore (NUS)
- NUHSRO/2021/034/TRP/09/Nanomedicine/National University of Singapore (NUS)
- (MOH-001388-00, CG21APR1005)/MOH | National Medical Research Council (NMRC)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

