Extracellular vesicles activated cancer-associated fibroblasts promote lung cancer metastasis through mitophagy and mtDNA transfer
- PMID: 38825680
- PMCID: PMC11145873
- DOI: 10.1186/s13046-024-03077-w
Extracellular vesicles activated cancer-associated fibroblasts promote lung cancer metastasis through mitophagy and mtDNA transfer
Abstract
Background: Studies have shown that oxidative stress and its resistance plays important roles in the process of tumor metastasis, and mitochondrial dysfunction caused by mitochondrial DNA (mtDNA) damage is an important molecular event in oxidative stress. In lung cancer, the normal fibroblasts (NFs) are activated as cancer-associated fibroblasts (CAFs), and act in the realms of the tumor microenvironment (TME) with consequences for tumor growth and metastasis. However, its activation mechanism and whether it participates in tumor metastasis through antioxidative stress remain unclear.
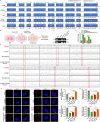
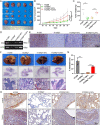
Methods: The role and signaling pathways of tumor cell derived extracellular vesicles (EVs) activating NFs and the characteristic of induced CAFs (iCAFs) were measured by the transmission electron microscopy, nanoparticle tracking analysis, immunofluorescence, collagen contraction assay, quantitative PCR, immunoblotting, luciferase reporter assay and mitochondrial membrane potential detection. Mitochondrial genome and single nucleotide polymorphism sequencing were used to investigate the transport of mtDNA from iCAFs to ρ0 cells, which were tumor cells with mitochondrial dysfunction caused by depletion of mtDNA. Further, the effects of iCAFs on mitochondrial function, growth and metastasis of tumor cells were analysed in co-culture models both in vitro and in vivo, using succinate dehydrogenase, glutathione and oxygen consumption rate measurements, CCK-8 assay, transwell assay, xenotransplantation and metastasis experiments as well as in situ hybridization and immunohistochemistry.
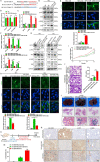
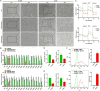
Results: Our findings revealed that EVs derived from high-metastatic lung cancer cells packaged miR-1290 that directly targets MT1G, leading to activation of AKT signaling in NFs and inducing NFs conversion to CAFs. The iCAFs exhibit higher levels of autophagy and mitophagy and more mtDNA release, and reactive oxygen species (ROS) could further promote this process. After cocultured with the conditioned medium (CM) of iCAFs, the ρ0 cells may restore its mitochondrial function by acquisition of mtDNA from CAFs, and further promotes tumor metastasis.
Conclusions: These results elucidate a novel mechanism that CAFs activated by tumor-derived EVs can promote metastasis by transferring mtDNA and restoring mitochondrial function of tumor cells which result in resistance of oxidative stress, and provide a new therapeutic target for lung cancer metastasis.
Keywords: Cancer-associated fibroblasts; Extracellular vesicles; Lung cancer; Metastasis; Mitophagy; mtDNA.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Laoukili J, van Schelven S, Kucukkose E, Verheem A, Goey K, Koopman M, Borel Rinkes I, Kranenburg O. BRAF(V600E) in colorectal cancer reduces sensitivity to oxidative stress and promotes site-specific metastasis by stimulating glutathione synthesis. Cell Rep. 2022;41:111728. doi: 10.1016/j.celrep.2022.111728. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

