The assessment of left ventricular diastolic function: guidance and recommendations from the British Society of Echocardiography
- PMID: 38825710
- PMCID: PMC11145885
- DOI: 10.1186/s44156-024-00051-2
The assessment of left ventricular diastolic function: guidance and recommendations from the British Society of Echocardiography
Abstract
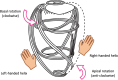
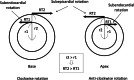
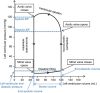
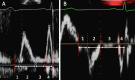
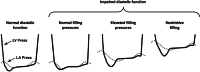
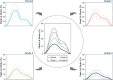
Impairment of left ventricular (LV) diastolic function is common amongst those with left heart disease and is associated with significant morbidity. Given that, in simple terms, the ventricle can only eject the volume with which it fills and that approximately one half of hospitalisations for heart failure (HF) are in those with normal/'preserved' left ventricular ejection fraction (HFpEF) (Bianco et al. in JACC Cardiovasc Imaging. 13:258-271, 2020. 10.1016/j.jcmg.2018.12.035), where abnormalities of ventricular filling are the cause of symptoms, it is clear that the assessment of left ventricular diastolic function (LVDF) is crucial for understanding global cardiac function and for identifying the wider effects of disease processes. Invasive methods of measuring LV relaxation and filling pressures are considered the gold-standard for investigating diastolic function. However, the high temporal resolution of trans-thoracic echocardiography (TTE) with widely validated and reproducible measures available at the patient's bedside and without the need for invasive procedures involving ionising radiation have established echocardiography as the primary imaging modality. The comprehensive assessment of LVDF is therefore a fundamental element of the standard TTE (Robinson et al. in Echo Res Pract7:G59-G93, 2020. 10.1530/ERP-20-0026). However, the echocardiographic assessment of diastolic function is complex. In the broadest and most basic terms, ventricular diastole comprises an early filling phase when blood is drawn, by suction, into the ventricle as it rapidly recoils and lengthens following the preceding systolic contraction and shortening. This is followed in late diastole by distension of the compliant LV when atrial contraction actively contributes to ventricular filling. When LVDF is normal, ventricular filling is achieved at low pressure both at rest and during exertion. However, this basic description merely summarises the complex physiology that enables the diastolic process and defines it according to the mechanical method by which the ventricles fill, overlooking the myocardial function, properties of chamber compliance and pressure differentials that determine the capacity for LV filling. Unlike ventricular systolic function where single parameters are utilised to define myocardial performance (LV ejection fraction (LVEF) and Global Longitudinal Strain (GLS)), the assessment of diastolic function relies on the interpretation of multiple myocardial and blood-flow velocity parameters, along with left atrial (LA) size and function, in order to diagnose the presence and degree of impairment. The echocardiographic assessment of diastolic function is therefore multifaceted and complex, requiring an algorithmic approach that incorporates parameters of myocardial relaxation/recoil, chamber compliance and function under variable loading conditions and the intra-cavity pressures under which these processes occur. This guideline outlines a structured approach to the assessment of diastolic function and includes recommendations for the assessment of LV relaxation and filling pressures. Non-routine echocardiographic measures are described alongside guidance for application in specific circumstances. Provocative methods for revealing increased filling pressure on exertion are described and novel and emerging modalities considered. For rapid access to the core recommendations of the diastolic guideline, a quick-reference guide (additional file 1) accompanies the main guideline document. This describes in very brief detail the diastolic investigation in each patient group and includes all algorithms and core reference tables.
Keywords: Diastolic function; Filling pressures; HFpEF; Left atrial pressure.
© 2024. The Author(s).
Conflict of interest statement
Otto A. Smiseth is co-inventor of “Method for myocardial segment work analysis”, has filed patent on “Estimation of blood pressure in the heart”, and has received one speaker honorarium from GE Healthcare. SR is co-Editor-in-Chief of Echo Research and Practice. LR, DA, DO, AH, MS-S and MP are Associate Editors of Echo Research and Practice. NS is a member of the Echo Research and Practice Editorial Board.
Figures










References
-
- Bianco CM, Farjo PD, Ghaffar YA, Sengupta PP. Myocardial mechanics in patients with normal LVEF and diastolic dysfunction. JACC Cardiovasc Imaging. 2020;13(1 Pt 2):258–271. 10.1016/j.jcmg.2018.12.035 - PubMed
-
- Robinson S, Rana B, Oxborough D, Steeds R, Monaghan M, Stout M, Pearce K, Harkness A, Ring L, Paton M, Akhtar W, Bedair R, Bhattacharyya S, Collins K, Oxley C, Sandoval J, Schofield MBChBR, Siva A, Parker K, Willis J, Augustine DX. A practical guideline for performing a comprehensive transthoracic echocardiogram in adults: the British Society of Echocardiography minimum dataset. Echo Res Pract. 2020;7(4):G59–G93. doi: 10.1530/ERP-20-0026. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

