Therapeutic patterns and migraine disease burden in switchers of CGRP-targeted monoclonal antibodies - insights from the German NeuroTransData registry
- PMID: 38825722
- PMCID: PMC11145812
- DOI: 10.1186/s10194-024-01790-7
Therapeutic patterns and migraine disease burden in switchers of CGRP-targeted monoclonal antibodies - insights from the German NeuroTransData registry
Abstract
Background: Monoclonal antibodies (mAbs) targeting the calcitonin gene-related peptide (CGRP) pathway have shown good efficacy in migraine prophylaxis. However, a subset of patients does not respond to the first mAb treatment and switches among the available mAbs. The goal of this study is to characterize the switching pattern of migraine patients treated with anti-CGRP(-receptor, -R) mAbs, and to describe the headache burden of those who did not switch, switched once, and switched twice.
Methods: This study used real world data from the NeuroTransData Cohort, a registry of migraine patients treated at outpatient neurology clinics across Germany. Patients who had received at least one anti-CGRP(-R) mAb were included. Headache diaries were collected at baseline and during treatment, along with quality of life measures every three months. Results were summarized for the subgroups of patients who did not switch and those with one and two switches.
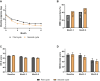
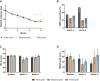
Results: Of the 655 eligible patients, 479 did not switch, 135 switched once, 35 twice, and 6 three or more times. The ≥ 50% response rates for monthly migraine days were 64.7%, 50.7%, and 25.0% for the no switch, one switch, and two switches groups in their last treatment cycles, respectively. Quality of life measures improved for the no switch and one switch groups, but not for the two switches group.
Conclusion: Patients who switched among anti-CGRP(-R) mAbs during the course of their treatment still benefited overall but to a lesser extent than those who did not switch. Treatment response in patients who switched twice was markedly lower compared to the no switch and one switch subgroup.
Keywords: Calcitonin-gene-related peptide; Migraine; Monoclonal antibody; Prophylaxis; Real-world experience.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Haghdoost F, Puledda F, Garcia-Azorin D, et al. Evaluating the efficacy of CGRP mAbs and gepants for the preventive treatment of migraine: a systematic review and network meta-analysis of phase 3 randomised controlled trials. Cephalalgia. 2023;43:033310242311593. doi: 10.1177/03331024231159366. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

