Cure can be achieved by conversion to microwave ablation following atezolizumab-bevacizumab therapy in unresectable hepatocellular carcinoma
- PMID: 38825875
- PMCID: PMC11449580
- DOI: 10.17998/jlc.2024.05.23
Cure can be achieved by conversion to microwave ablation following atezolizumab-bevacizumab therapy in unresectable hepatocellular carcinoma
Abstract
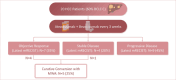
Backgrounds/aims: Atezolizumab/bevacizumab is the recommended first-line systemic therapy for unresectable hepatocellular carcinoma (uHCC) and may facilitate curative conversion through resection and locoregional therapies. However, there have been very few reports on curative conversion using microwave ablation (MWA). This study aimed to determine the curative conversion rate with MWA using atezolizumab-bevacizumab as the first-line treatment in patients with uHCC, and to compare the characteristics and survival of patients with and without curative conversion.
Methods: Consecutive patients with uHCC who were started on atezolizumab-bevacizumab from May 2021 to December 2023 in a single tertiary center were included. Objective response rate (ORR) and disease control rate (DCR) were based on the Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 and modified RECIST (mRECIST) criteria.
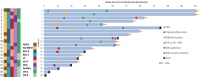
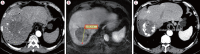
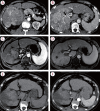
Results: Twenty consecutive patients with uHCC (60% advanced-stage) were included, 90% exceeding the up-to-7 criteria. The ORR and DCR were 35% and 60%, 35% and 55% using RECIST and mRECIST, respectively. Five patients (25%) underwent successful curative conversion with MWA (four advanced and one intermediate stage) despite a median HCC size of 6.1 cm (range, 2.4-7.3). Two of these patients were tumor and drug-free 132-133 weeks from the 1st atezolizumab-bevacizumab dose. Patients who underwent curative conversion had significantly longer survival than those who did not (P=0.024). Other factors associated with survival were male sex, Child-Pugh class A, and an objective response.
Conclusions: Despite the relatively large tumor size, successful curative conversion with MWA was achieved with first-line atezolizumab-bevacizumab in uHCC. However, data from prospective multicenter trials are required to determine whether this strategy is universally applicable.
Keywords: Combination; Immune checkpoint inhibitors; Locoregional therapy; Microwave ablation; Tyrosine kinase inhibitors.
Conflict of interest statement
The authors did not receive any financial assistance for the work. S. Wong is a lecturer for Roche and Hi-Eisai. No potential conflicts of interests exist for all other authors.
Figures

References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Abou-Alfa GK, Chan SL, Kudo M, Lau G, Kelley RK, Furuse J, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40 Suppl 4:379.
-
- Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. - PubMed
LinkOut - more resources
Full Text Sources

