This is a preprint.
Liquid-like condensates that bind actin drive filament polymerization and bundling
- PMID: 38826190
- PMCID: PMC11142076
- DOI: 10.1101/2024.05.04.592527
Liquid-like condensates that bind actin drive filament polymerization and bundling
Update in
-
Liquid-like condensates that bind actin promote assembly and bundling of actin filaments.Dev Cell. 2025 Jun 9;60(11):1550-1567.e4. doi: 10.1016/j.devcel.2025.01.012. Epub 2025 Feb 5. Dev Cell. 2025. PMID: 39914390
Abstract
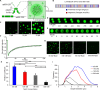
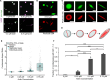
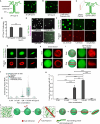
Liquid-like protein condensates perform diverse physiological functions. Previous work showed that VASP, a processive actin polymerase, forms condensates that polymerize and bundle actin. To minimize their curvature, filaments accumulated at the inner condensate surface, ultimately deforming the condensate into a rod-like shape, filled with a bundle of parallel filaments. Here we show that this behavior does not require proteins with specific polymerase activity. Specifically, we found that condensates composed of Lamellipodin, a protein that binds actin but is not an actin polymerase, were also capable of polymerizing and bundling actin filaments. To probe the minimum requirements for condensate-mediated actin bundling, we developed an agent-based computational model. Guided by its predictions, we hypothesized that any condensate-forming protein that binds actin could bundle filaments through multivalent crosslinking. To test this idea, we added an actin-binding motif to Eps15, a condensate-forming protein that does not normally bind actin. The resulting chimera formed condensates that drove efficient actin polymerization and bundling. Collectively, these findings broaden the family of proteins that could organize cytoskeletal filaments to include any actin-binding protein that participates in protein condensation.
Figures



Similar articles
-
Liquid-like condensates that bind actin promote assembly and bundling of actin filaments.Dev Cell. 2025 Jun 9;60(11):1550-1567.e4. doi: 10.1016/j.devcel.2025.01.012. Epub 2025 Feb 5. Dev Cell. 2025. PMID: 39914390
-
Liquid-like VASP condensates drive actin polymerization and dynamic bundling.Nat Phys. 2023 Apr;19(4):574-585. doi: 10.1038/s41567-022-01924-1. Epub 2023 Jan 30. Nat Phys. 2023. PMID: 38405682 Free PMC article.
-
Liquid-like condensates mediate competition between actin branching and bundling.bioRxiv [Preprint]. 2023 Jun 26:2023.06.23.546267. doi: 10.1101/2023.06.23.546267. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2024 Jan 16;121(3):e2309152121. doi: 10.1073/pnas.2309152121. PMID: 37425724 Free PMC article. Updated. Preprint.
-
Synaptopodin family of natively unfolded, actin binding proteins: physical properties and potential biological functions.Biophys Rev. 2010 Dec;2(4):181-189. doi: 10.1007/s12551-010-0040-5. Epub 2010 Nov 20. Biophys Rev. 2010. PMID: 28510039 Free PMC article. Review.
-
Biomolecular condensation involving the cytoskeleton.Brain Res Bull. 2023 Mar;194:105-117. doi: 10.1016/j.brainresbull.2023.01.009. Epub 2023 Jan 20. Brain Res Bull. 2023. PMID: 36690162 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
